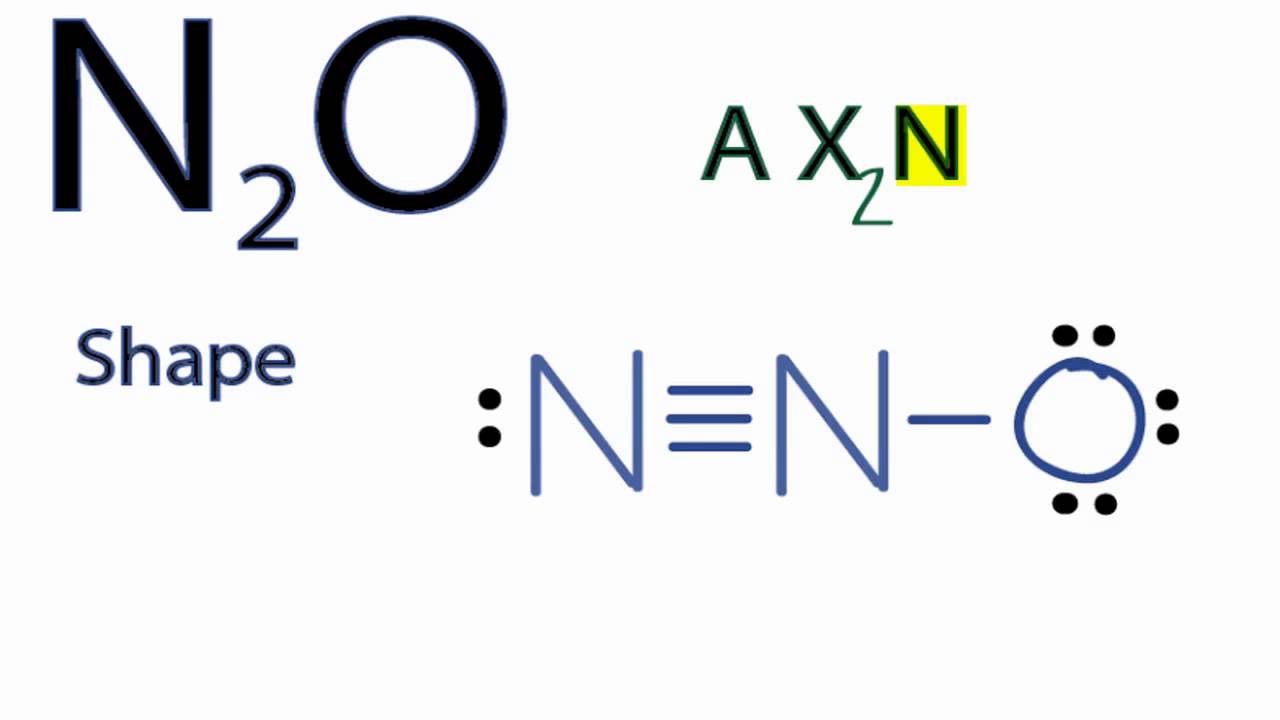
N2O Molecular Geometry / Shape and Bond Angles YouTube
This chemistry video tutorial explains how to draw the lewis structure of N2O also known as Nitrous Oxide or Dinitrogen Monoxide. It also covers the molecul.

How to draw NO2+ Lewis Structure? Science Education and Tutorials
The Lewis electron structure for the NH 4+ ion is as follows: The nitrogen atom shares four bonding pairs of electrons, and a neutral nitrogen atom has five valence electrons. Using Equation 4.4.1, the formal charge on the nitrogen atom is therefore. formalcharge(N) = 5 −(0 + 8 2) = 0.

N2O Lewis Structure Nitrous Oxide YouTube
Explanation: 16 valence electrons: 2 × 5nitrogen + 1 × 6oxygen = 8 electron pairs..to distribute over 3 centres. And you must simply KNOW here that the oxygen is terminal. And given the example, there will be formal charge separation. N ≡ + N − O− versus −N = + N = O.

NO2 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Diagram Techiescientist
Understanding the Lewis Structure of N2O. Nitrous Oxide (N 2 O) is a compound commonly encountered in Chemistry and plays a significant role in Mathematics education. This section provides a detailed explanation of the Lewis structure of N 2 O and its importance in understanding molecular geometry.. The Role of Electron Pair Repulsion Theory

[DIAGRAM] Kcl Lewis Dot Diagram
For N2O, we add up the valence electrons of nitrogen (5) and the two oxygen atoms (6 each), giving us a total of 16 valence electrons. Place the atoms around the central atom: In the N2O Lewis structure, we arrange the two oxygen atoms around the central nitrogen atom. We then connect the atoms with single bonds.
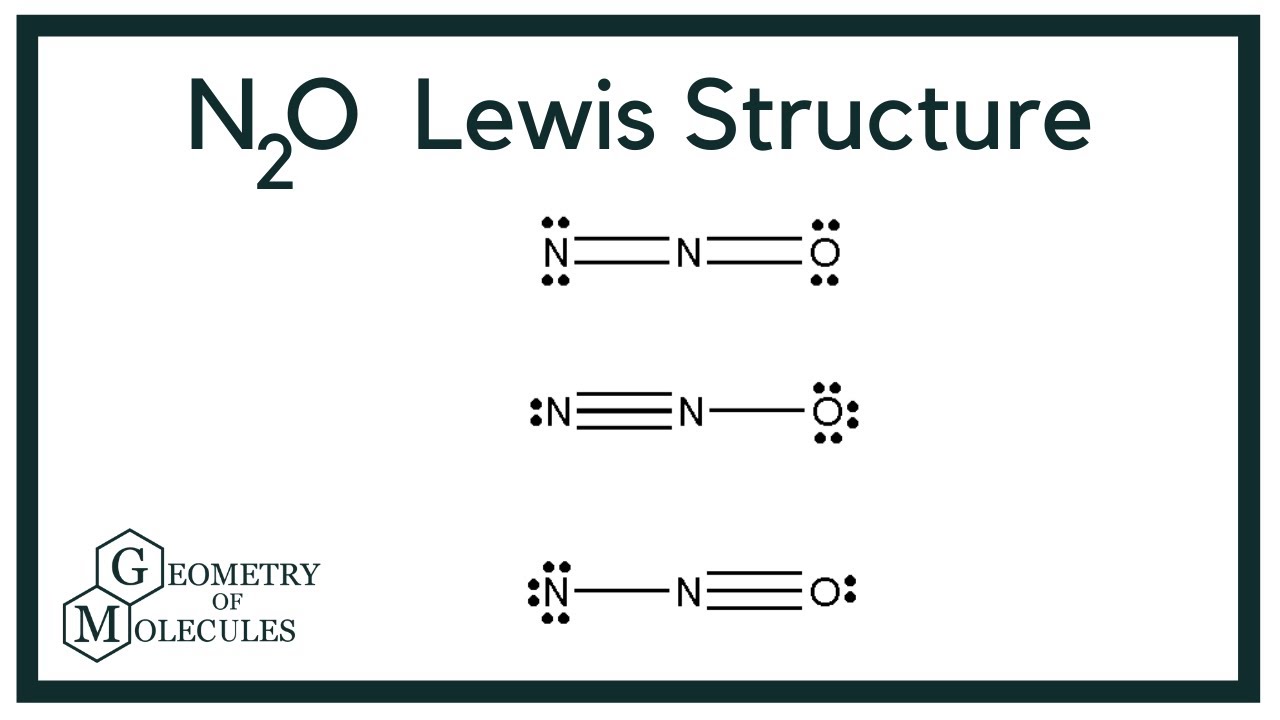
N2O Lewis Structure (Dinitrogen Oxide) YouTube
MUNICH, October 28, 2021: Stuttgart Airport is expected to become a hub for regional electric flights with zero operating emissions, following the agreement between Stuttgart Airport and Lilium. Stuttgart is joining the planned southern German network which already consists of the Munich and Nuremberg airports, as previously announced.The Munich-based aviation company Lilium, positioned to be.

N2O Lewis Structure YouTube
Lewis structure of a water molecule. Lewis structures - also called Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDs) - are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as.

QUIMICA Estructura de Lewis de N2O y carga formal AULAEXPRESS YouTube
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".

N2o Estructura De Lewis Balan
Here's how you can easily draw the N 2 O Lewis structure step by step: #1 Draw a rough skeleton structure. #2 Mention lone pairs on the atoms. #3 If needed, mention formal charges on the atoms. #4 Minimize formal charges by converting lone pairs of the atoms, and try to get a stable Lewis structure.

Dengan menggunakan rumus titik elektron (struktur
A step-by-step explanation of how to draw the N2O Lewis Dot Structure (Dinitrogen monoxide or Nitrous Oxide).For the N2O structure use the periodic table to.

Drawing Lewis Structures Chemistry Socratic
The Lewis structure of N2O, also known as nitrous oxide, involves two nitrogen atoms (N) and one oxygen atom (O). Nitrogen has 5 valence electrons, while oxygen has 6 valence electrons. The total number of valence electrons in N2O is calculated as follows: 2 (N) + 1 (O) = 2 (5) + 1 (6) = 10 + 6 = 16 valence electrons.

N2O Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram Techiescientist
Learn about TEAM LEWIS Munich (Germany) office. Search jobs. See reviews, salaries & interviews from TEAM LEWIS employees in Munich (Germany).
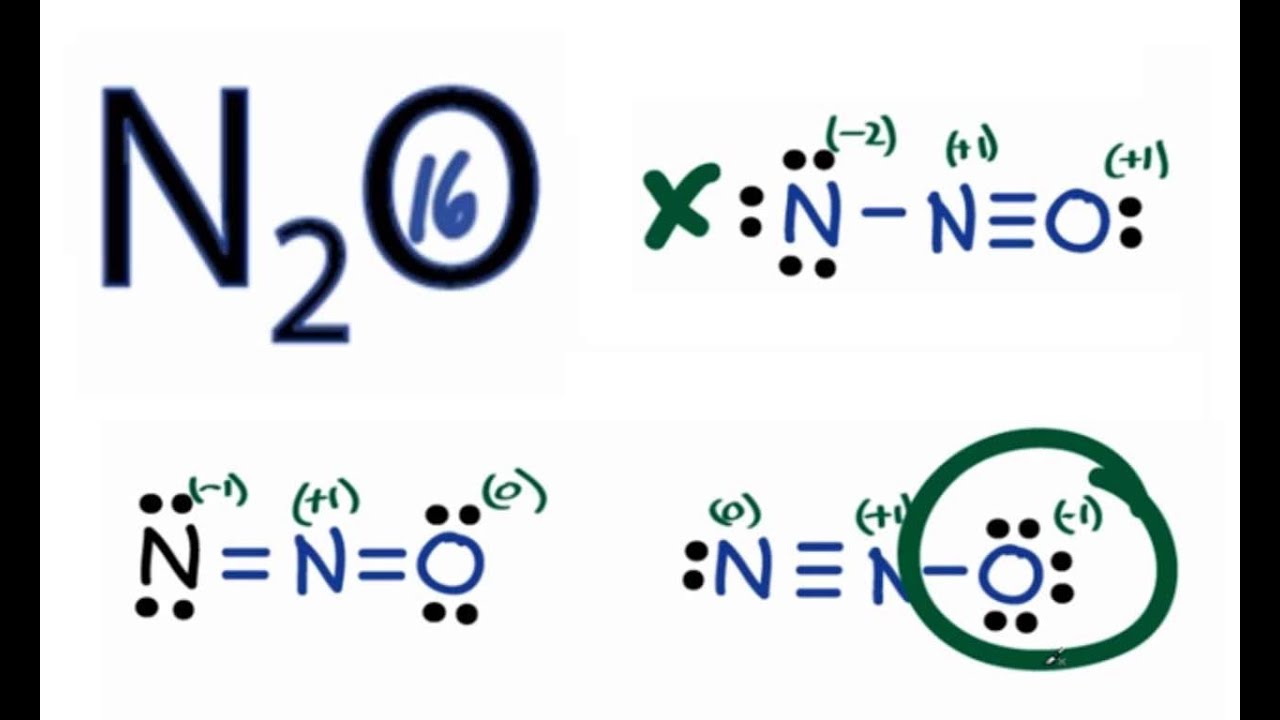
N2O Lewis Structure How to Draw the Lewis Structure for N2O YouTube
There are several resonance structures for N2O (Nitrous oxide). We start with a valid Lewis structure and then follow these general rules. For N2O resonance.

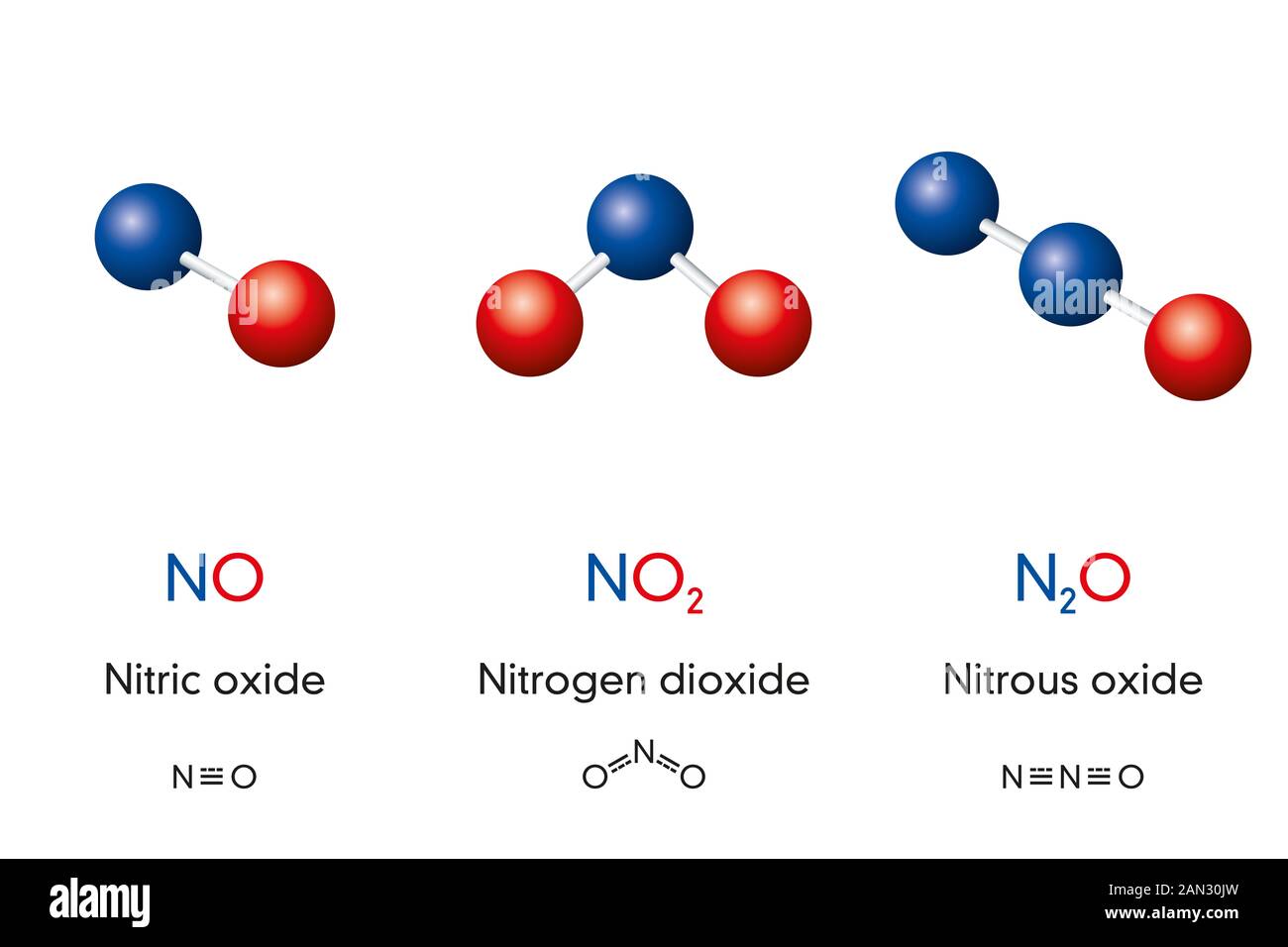
Chemical formulas and molecule model of nitrogen oxide nitric oxide NO, nitrogen dioxide NO2
Step #1: Calculate the total number of valence electrons. Here, the given molecule is N2O. In order to draw the lewis structure of N2O, first of all you have to find the total number of valence electrons present in the N2O molecule. (Valence electrons are the number of electrons present in the outermost shell of an atom).
N2o Name
Steps of drawing N2O lewis structure Step 1: Find the total valence electrons in N2O molecule. In order to find the total valence electrons in N2O molecule, first of all you should know the valence electrons present in the nitrogen atom as well as oxygen atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.). Here, I'll tell you how you can easily.

N2o nitrous oxide molecule Royalty Free Vector Image
N2O or nitrous oxide is commonly known as laughing gas. There are several other names by which this compound is known like sweet air, protoxide of nitrogen, etc. N2O is a colorless gas with a molecular weight of 44.013 g/mol. The boiling point of this compound is -88.48℃ and the melting point is -90.86℃.