Lewis Dot Structure Of H3po4 Phosphoric Acid
Step #1: Calculate the total number of valence electrons. Here, the given molecule is H3PO4 (phosphoric acid). In order to draw the lewis structure of H3PO4, first of all you have to find the total number of valence electrons present in the H3PO4 molecule. (Valence electrons are the number of electrons present in the outermost shell of an atom).

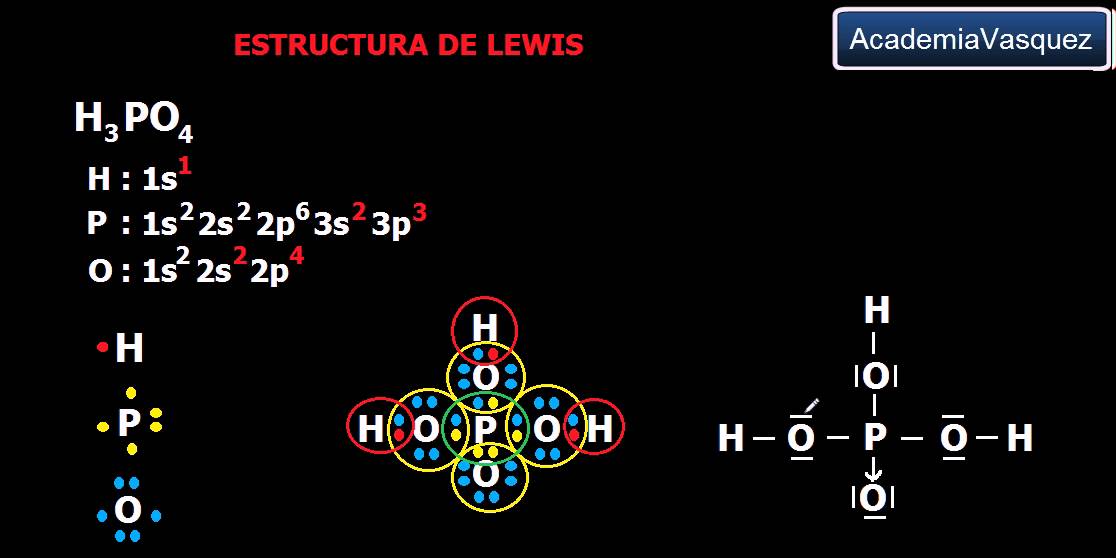
Estructura De Lewis Del Acido Fosforico Compuesto
PDF | On Feb 1, 2003, NIGEL CHAFFEY published Alberts, B., Johnson, A., Lewis, J., Raff, M., Roberts, K. and Walter, P. Molecular biology of the cell. 4th edn. | Find.

rumus lewis dari H3PO4 Brainly.co.id
In the H 3 PO 4 Lewis structure Phosphorous (P) is least electron electronegative atom and goes in the center of the Lewis structure. When we have an H (or H2 or H3) in front of a polyatomic molecule (like CO 3, PO 4, NO 2, etc.) we know that it's an acid. This means that the Hydrogen atoms will be attached to the outside of the oxygen molecules.
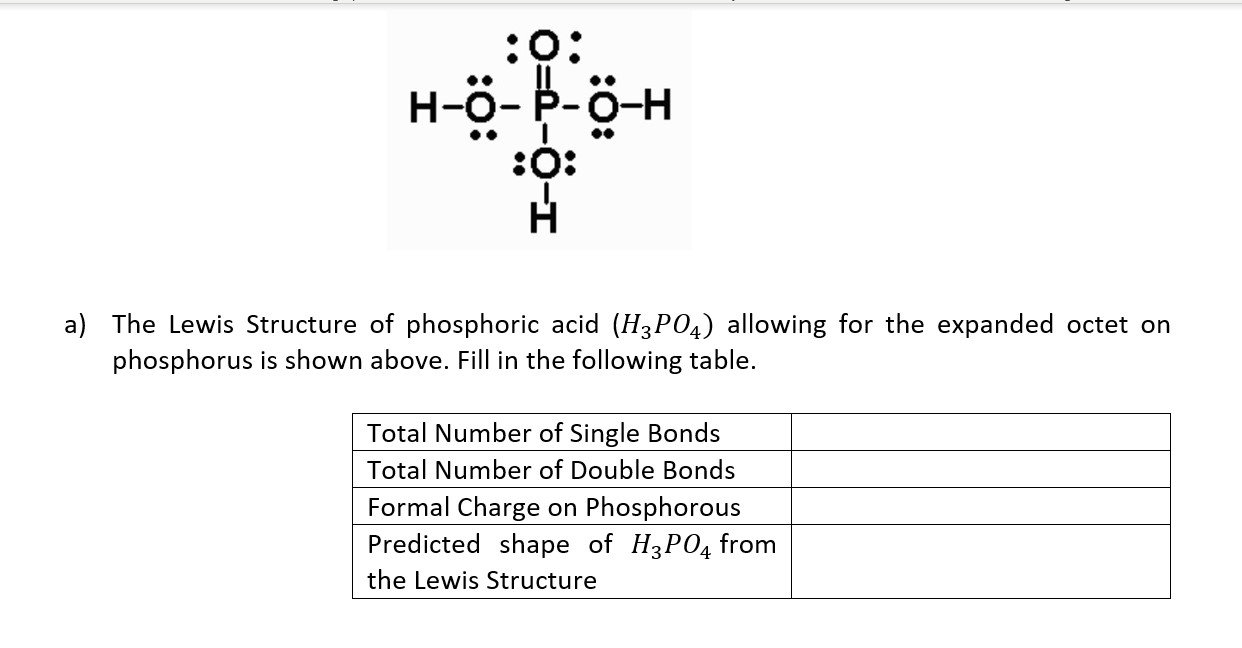
SOLVED a) The Lewis Structure of phosphoric acid (H3PO4) allowing for the expanded octet on
H 3 PO 4: Phosphates and Phosphoric Acid - Raw Materials, Technology, and Economics of the Wet Process H 3 PO 4.Von P. Becker. Marcel Dekker, Inc., New York - Basel 1983. 608 S., SFr. 253,-

How To Draw The Lewis Structure of Phosphoric Acid (H3PO4) YouTube
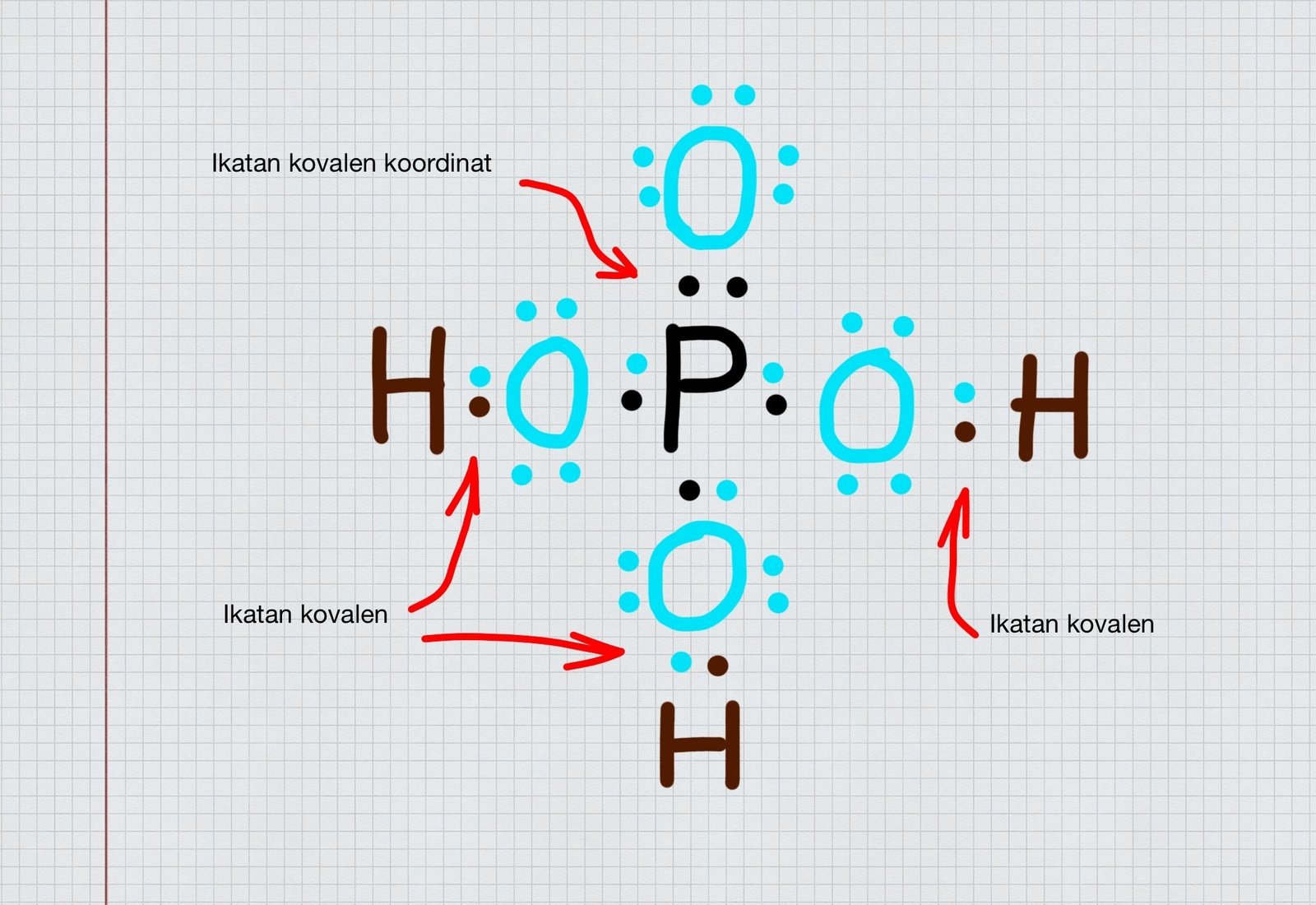
Pertanyaan. Perhatikan gambar struktur Lewis Senyawa H 3PO4 berikut! Pasangan elektron yang terbentuk secara kovalen koordinasi ditunjukkan pada nomor. (Nomor atom H = 1 ; O = 8 ; P = 15) 1. 2.

Gambarkan rumus titik elektron (struktur Lewis) dari molekulmolekul berikut YouTube
Steps of drawing H3PO4 lewis structure Step 1: Find the total valence electrons in H3PO4 molecule. In order to find the total valence electrons in H3PO4 (phosphoric acid) molecule, first of all you should know the valence electrons present in hydrogen atom, phosphorus atom as well as oxygen atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.)

H3PO4 Lewis Structure, Molecular Geometry, Hybridization, and Polarity Techiescientist
Pengertian Struktur Lewis. Struktur lewis atau sering disebut rumus lewis adalah suatu pola atau diagram yang menggambarkan jumlah elektron valensi dari atom-atom yang akan membentuk ikatan kimia. Struktur lewis ini berbentuk titik, silang atau bulatan-bulatan yang mengelilingi lambang atomnya, baik atom tunggal maupun atom-atom yang berikatan.

Phosphoric acid h3po4 Royalty Free Vector Image
Biochemistry and Molecular Biology Education is an international journal aimed to enhance teacher preparation and student learning in Biochemistry, Molecular Biology, and related sciences such as Biophysics and Cell Biology, by promoting the world-wide dissemination of educational materials.

Which is the Lewis structure for H3PO4?
44. Lewis Dot Structure of H3PO4 | How to Draw Lewis Structures| Class 11 Chemistry | Chemical BondingQueries Solved in this videos:-1) lewis structure2) lew.

H3PO4 Lewis Structure How to Draw the Lewis Structure for H3PO4 YouTube
4. (Phosphoric Acid) Phosphoric acid (H 3 PO 4) lewis structure is drawn using the concept of total valence electrons. H 3 PO 4 contains three elements phosphorous, oxygen and hydrogen. These elements provide electrons (not all electrons) of last shell to form bonds. In this tutorial, we will learn, how to construct the lewis structure of H 3.

H3po4
Lewis Structures: Lewis structures allow us to determine the shape of a molecule. We count the number of valence electrons required for the molecule and try to fill the octet for each constituent molecule while not adding or taking any electrons away from the molecule. Then we can use VSEPR theory to find the molecule's shape.
H3po4
H3PO4 is a chemical formula for Phosphoric acid and it is classified as a weak acid. This compound is also known as orthophosphoric acid. In this video we wi.

Bau der DNA
H 3 PO 4 (phosphoric acid) has three hydrogen atoms, one phosphorus atom, and four oxygen atoms.. In the H 3 PO 4 Lewis structure, there is one double bond and three single bonds around the phosphorus atom, with four oxygen atoms attached to it. The oxygen atom with a double bond has two lone pairs, and the left oxygen, right oxygen, and the bottom oxygen atom (with which the hydrogen atom is.

H3po4
About this video -Structure of Phosphoric acid H3PO4

H3PO4 Lewis Structure (Phosphoric Acid) YouTube
Start typing, then use the up and down arrows to select an option from the list.?
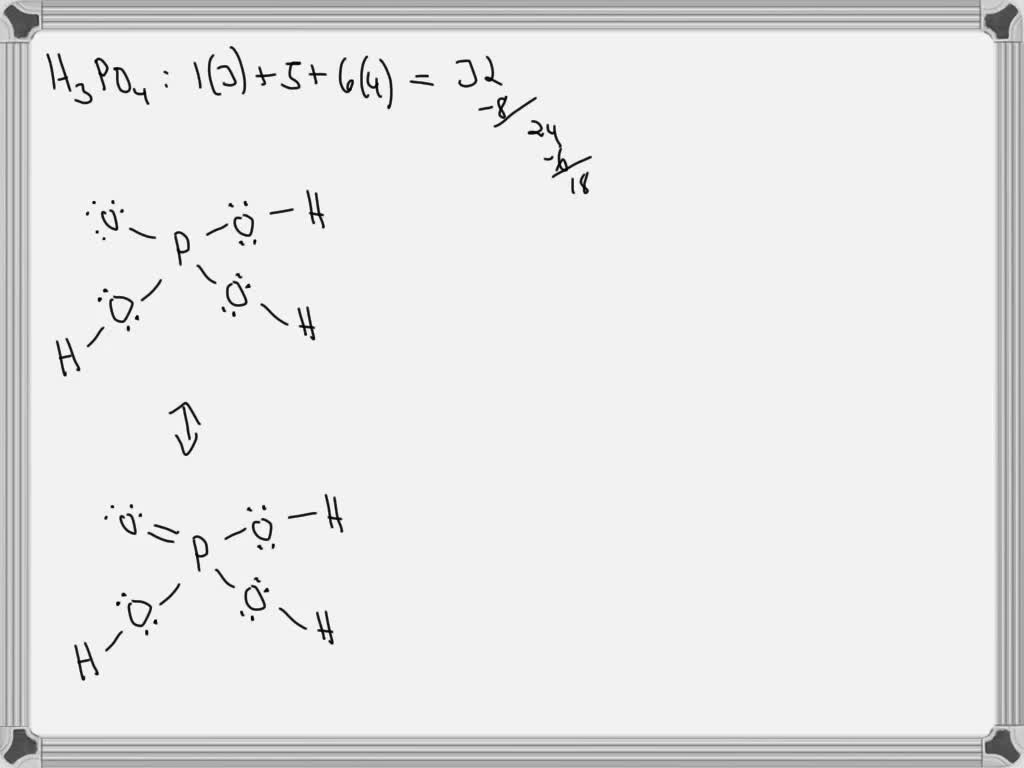
SOLVED Draw out the Lewis Structure of H3PO4, and consider its major resonance forms. Box the
A step-by-step explanation of how to draw the H3PO4 Lewis Dot Structure (Phosphoric acid).For the H3PO4 structure use the periodic table to find the total nu.