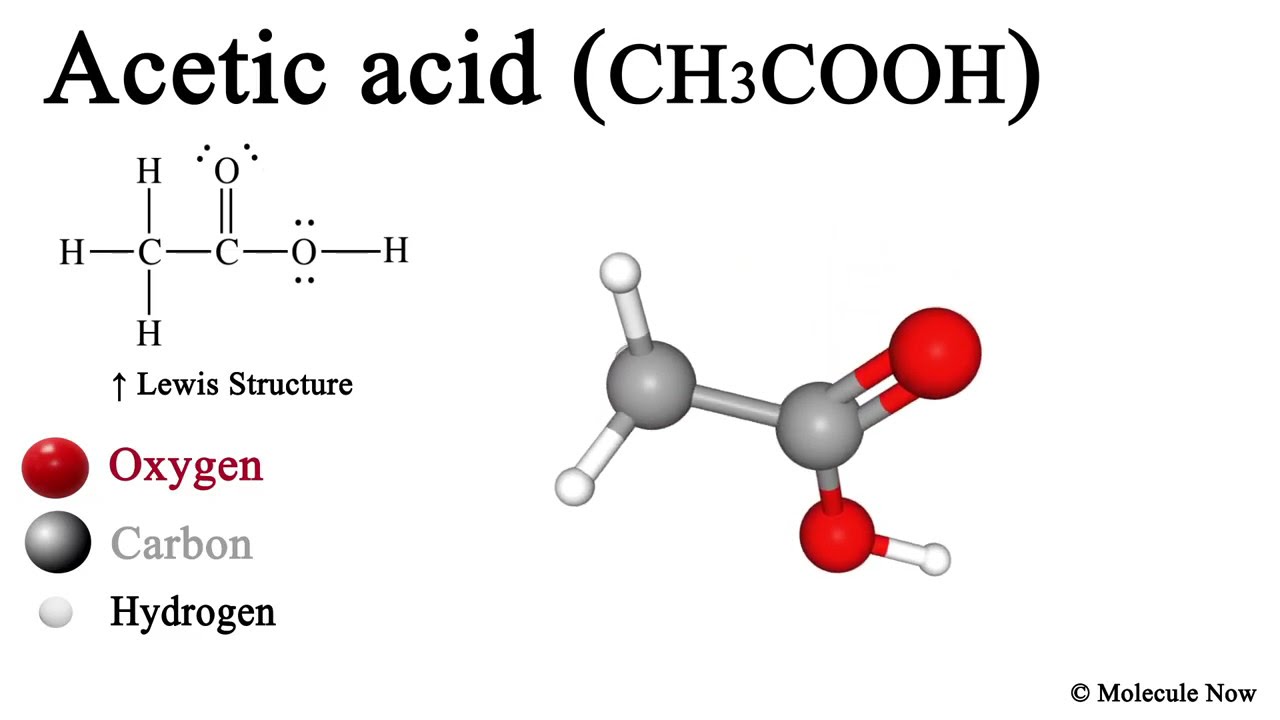
What Is Ch3cooh Lewis Structure?
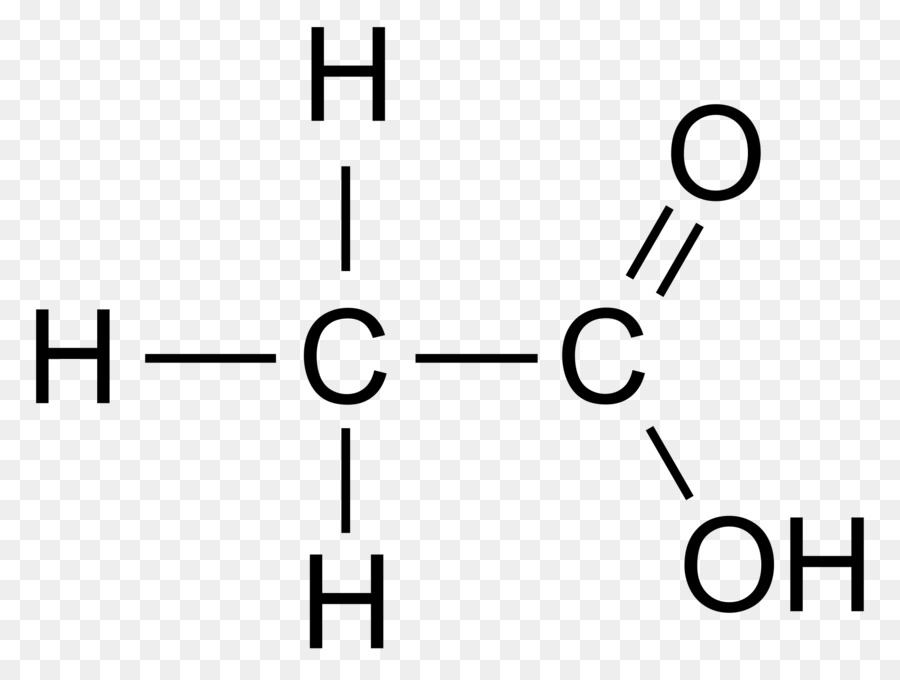
CH 3 COOH (acetic acid) has two carbon atoms, four hydrogen atoms, and two oxygen atoms.. In the CH 3 COOH Lewis structure, there is a single bond between the two carbon atoms. The left carbon is attached with three hydrogen atoms, and the right carbon is attached with two oxygen atoms. One oxygen atom makes a double bond with the carbon atom, and the other oxygen atom (with which the hydrogen.

The skeletal structure of `CH_(3)COOH` as shown below is correct, but some of the bonds are
In the Lewis structure of CH3COOH, the central atom is carbon (C), which is bonded to the oxygen atoms and hydrogen atom. 4. How many bonds does carbon form in the CH3COOH molecule? Acetic acid (CH3COOH) has 7 σ (sigma) bonds and 1 π (pi) bond. The π bond is formed between one of the oxygen atoms and the carbon atom.
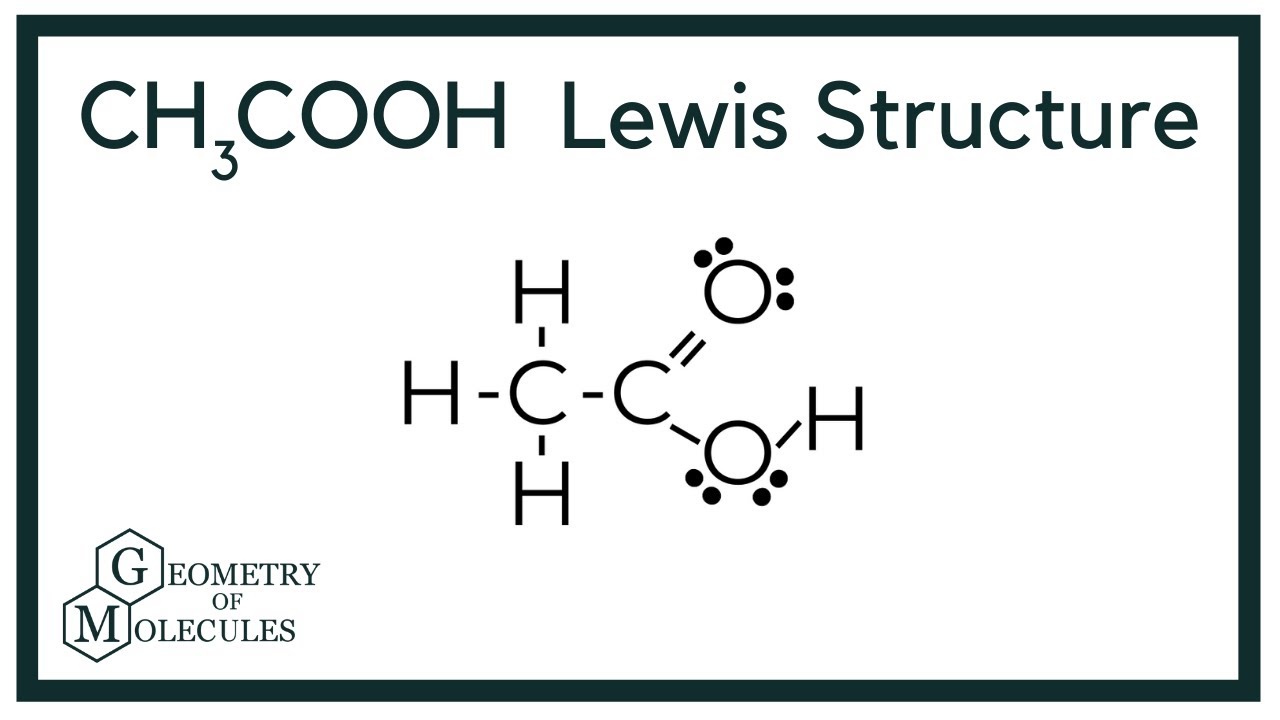
CH3COOH Lewis Structure (Acetic acid) YouTube
In this example, we draw the Lewis structure for the organic molecule CH3COOH, acetic acid, and evaluate it using formal charge.

[初三化学]CH3COOH为什么是酸不是碱?_百度知道
Lewis Publishers, Boca Raton, FL. 2000., p. 650 Hazardous Substances Data Bank (HSDB) When dogs were administered large doses (1-2 g/kg ip or sc) of sodium acetate , only small amounts appeared in the urine, which is evidence of the rapid utilization of acetic acid.

Lewis Structure For Ch3coo
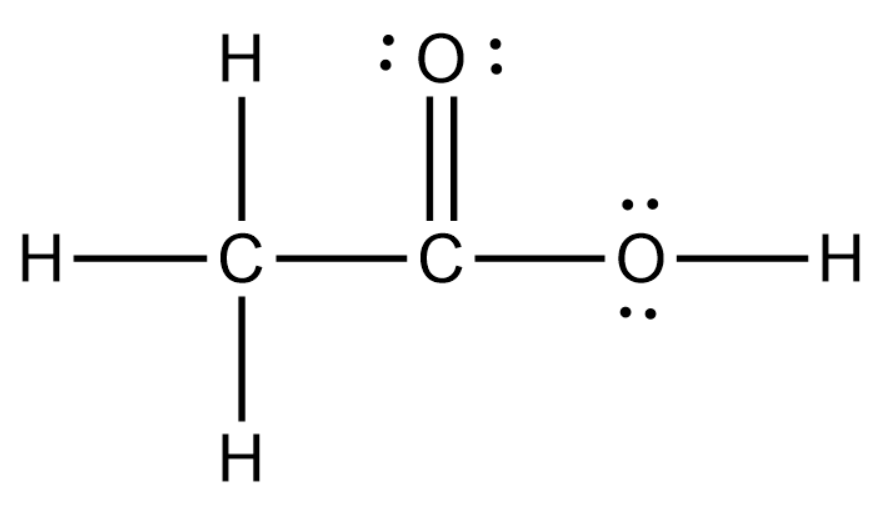
What is the Lewis structure of acetic acid? The Lewis structure of acetic acid (CH3COOH) consists of two carbon (C) atoms double-bonded to one oxygen (O) atom and a single bond to another oxygen atom. Each carbon atom is also bonded to three hydrogen (H) atoms, and the remaining oxygen atom has two lone pairs of electrons.. To draw the Lewis structure of acetic acid CH 3 COOH, follow these.
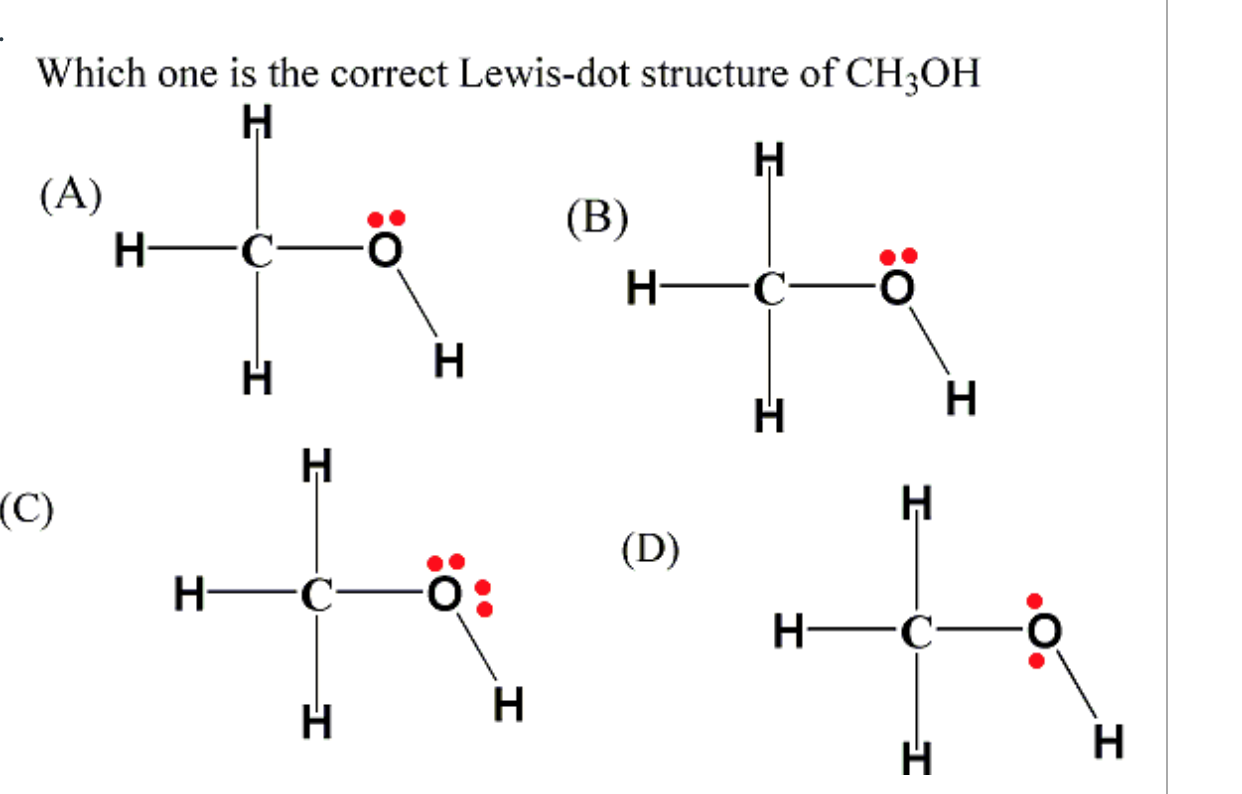
Lewis Structure Of H3coh
Steps of drawing CH3COOH lewis structure Step 1: Find the total valence electrons in CH3COOH molecule. In order to find the total valence electrons in a CH3COOH molecule, first of all you should know the valence electrons present in carbon atom, hydrogen atom as well as oxygen atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.)
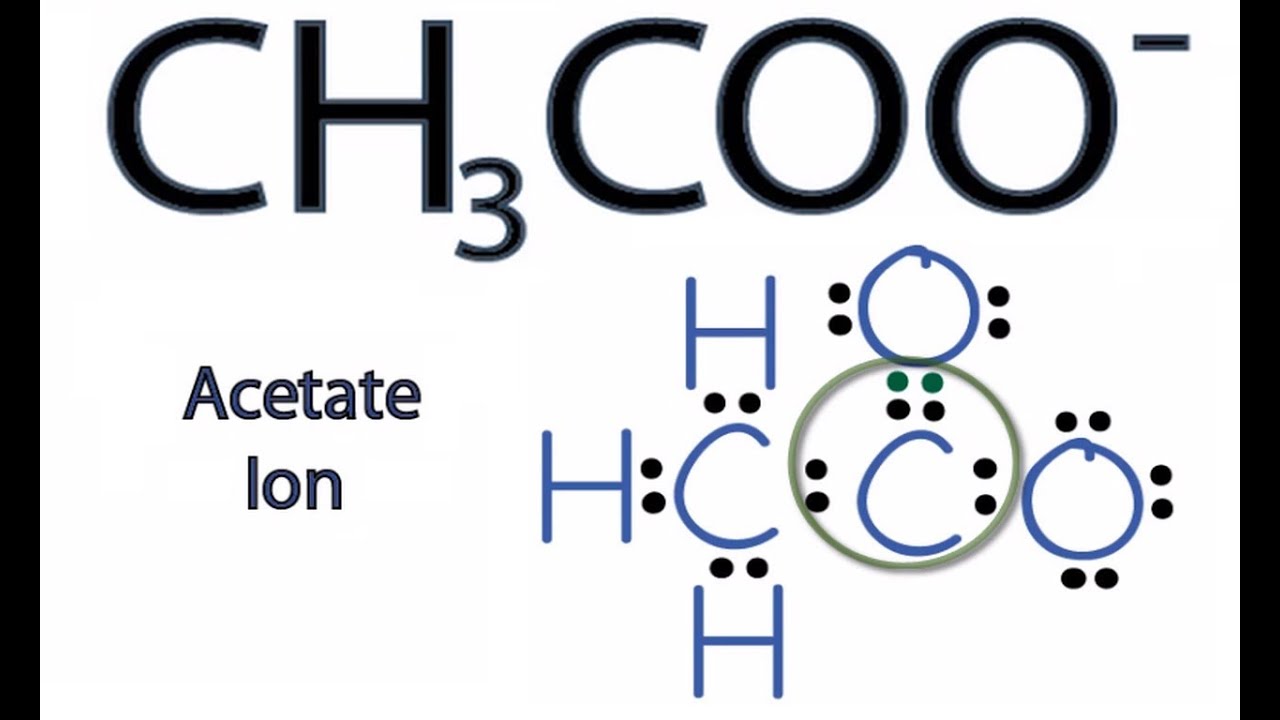
CH3COO Lewis Structure How to Draw the Lewis Structure for CH3COO YouTube
CH3OH as a Lewis Base. In addition to its role as a solvent and fuel, CH3OH, also known as methanol, can act as a Lewis base in certain chemical reactions. A Lewis base is a molecule or ion that donates a pair of electrons to form a coordinate bond with a Lewis acid. In the case of CH3OH, it can donate a lone pair of electrons from the oxygen atom.
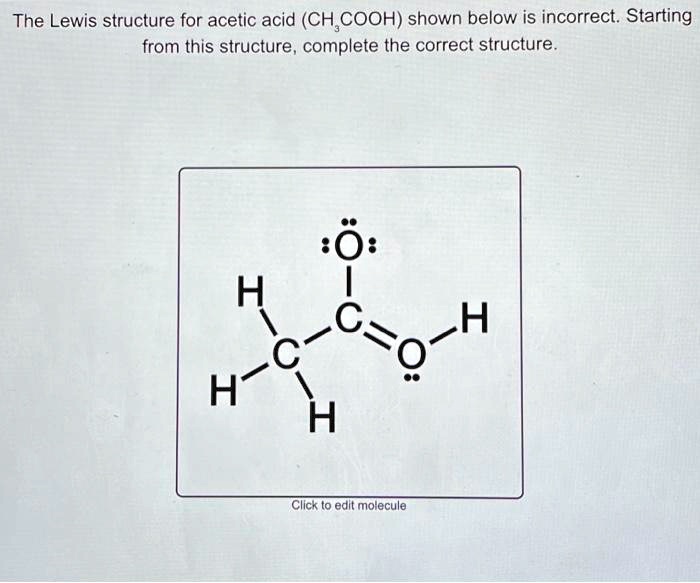
SOLVED The Lewis structure for acetic acid (CH3COOH) shown below is incorrect. Starting from
CH3COOH is a polar molecule in nature because of the unequal distribution of charge on the atom that leads to some net dipole moment. In acetic acid lewis structure, there are 3 C-H bonds, 1 C=O. bond, 1 C-O bond, 1 O-H bond and 1 C-C bond. CH3COOH has two types of molecular geometry or shape - Trigonal planar and Tetrahedral geometry.
อัลบั้ม 94+ ภาพพื้นหลัง Ch3cooh โครงสร้าง อัปเดต
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".
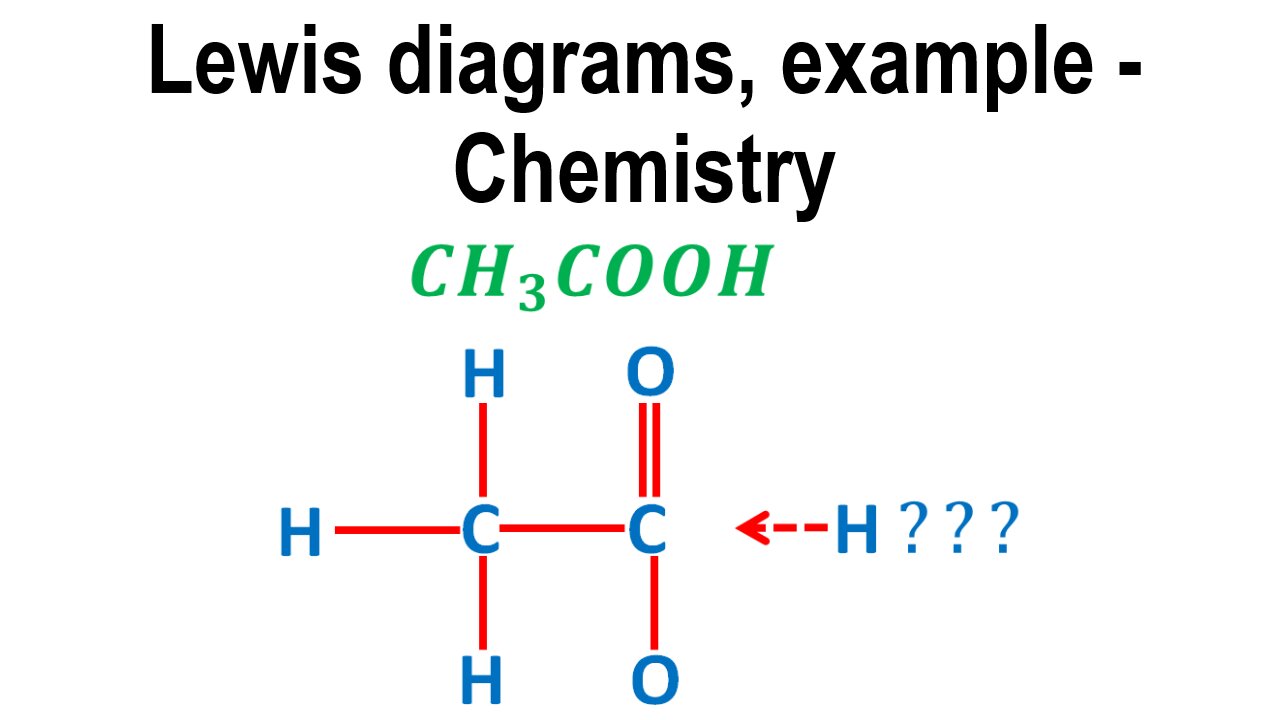
Lewis diagrams, acetic acid, CH3COOH, example Chemistry
A step-by-step explanation of how to draw the CH3COOH Lewis Dot Structure (Acetic acid).For the CH3COOH structure use the periodic table to find the total nu.

식초, 베이킹소다 반응 CH3COOH + NaHCO3
Drawing the Lewis Structure for CH 3 COOH (Acetic Acid). Viewing Notes: CH 3 COOH is an organic compound and the COOH is the carboxylic acid functional group. If you recognize this the Lewis structure is much easier to draw. In CH 3 COOH you should memorize what the COOH functional group looks like.; Remember that Hydrogen only needs 2 valence electrons for a full outer shell.

Ch3cooh Molecular Geometry
Lewis Structure Finder. This widget gets the Lewis structure of chemical compounds. Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.

QUIMICA Estructura de Lewis Acido Acético (CH3COOH) Carga Formal Hibridación AULAEXPRESS YouTube
Transcript: For the CH3COO- Lewis structure, we have a total of 24 valence electrons. We'll put the Carbons next to each other. We'll put an Oxygen on the end here, and we'll put another Oxygen here. Then we have those three Hydrogens, which we'll place around the Carbon on the end. We have 24 valence electrons for the CH3COOH- Lewis structure.
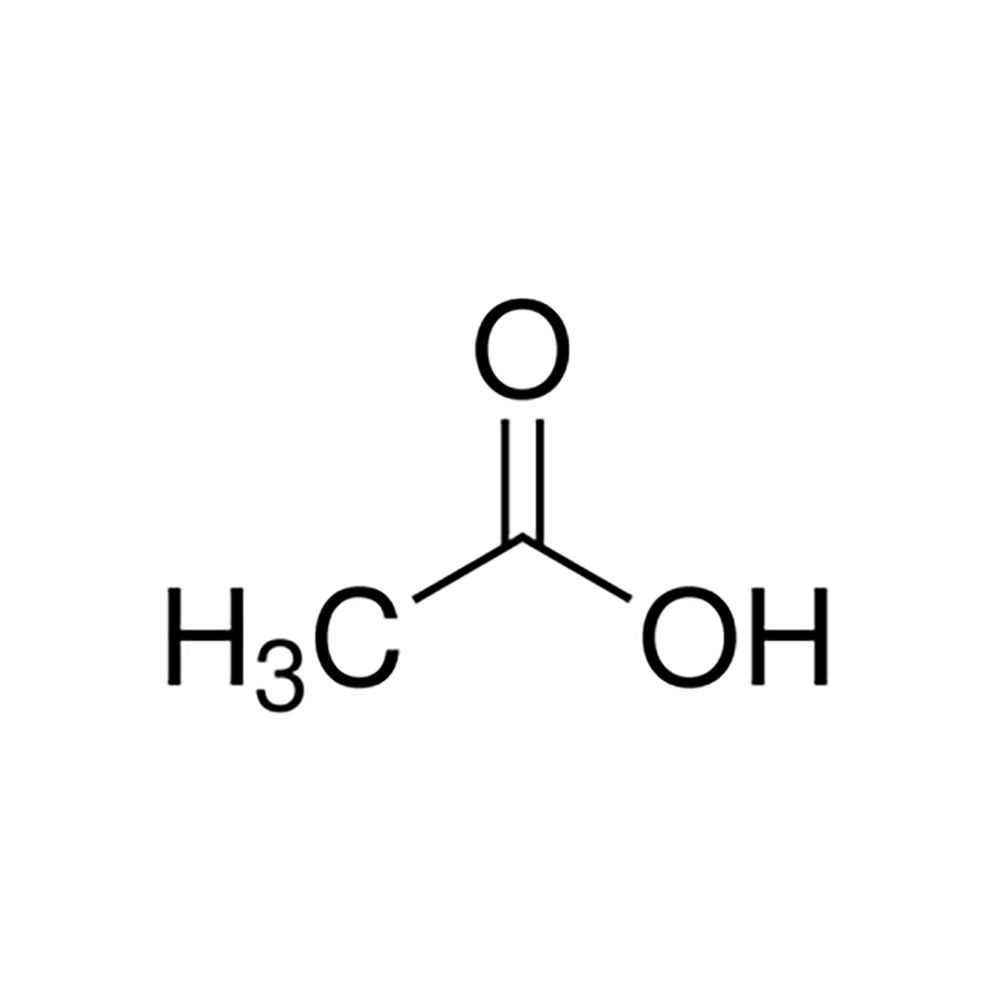
Acetic Acid Overview, Structure, Properties & Uses
CH3OH Lewis Structure. Lewis dot structure is a pictorial representation of the molecule, it's bonding with other atoms and the arrangement of atoms in the compound. It helps in knowing the number of bonded electrons, lone pairs, and the compound's molecular shape. Valence electrons help in drawing this Lewis structure, as all the electrons.
Struktur Lewis, Asam Asetat, Asetaldehida gambar png
A step-by-step explanation of how to draw the CH3COO- Lewis Dot Structure (Acetate ion).For the CH3COO- structure use the periodic table to find the total nu.

Chemistry Class 11 NCERT Solutions Chapter 4 Chemical Bonding and Molecular Structure Part 4
I quickly take you through how to draw the Lewis Structure of CH3COOH (Acetic Acid). I also go over hybridization, shape, sigma, pi bonding and bond angles.