
Qcp File Format Used Cellular Telephone Providing Ringtones Record Voice Stock Vector Image by
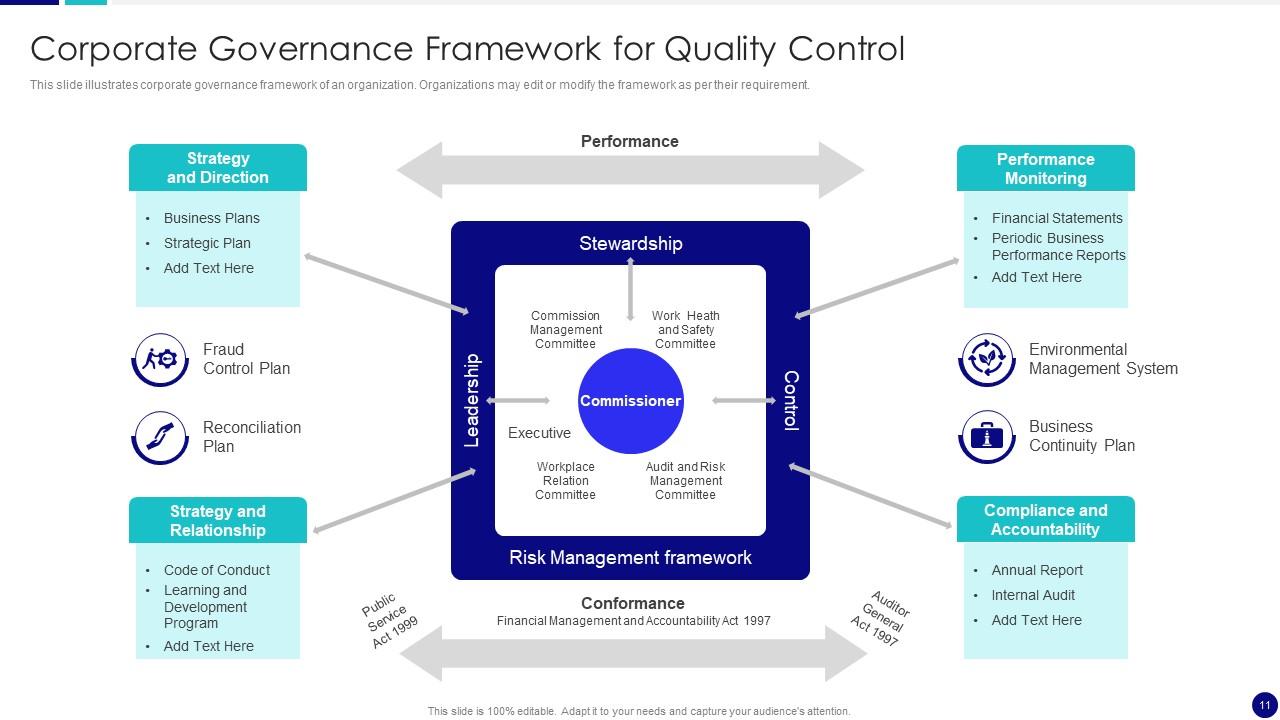
Th US Centers for Medicare and Medicaid Services (CMS) introduced a new option for Quality Control (QC) in 2013. There are only two options approved by CMS (CLIA) as of January, 2016: develop an Individualized Quality Control Plan (IQCP) or follow the traditional CLIA regulation for QC. IQCP is focused on risk mitigation and allows labs to.

Why is QCP Important for Architects? AWIQCP
Sedangkan QCP adalah program yang dirancang untuk memastikan bahwa proses produksi atau pelayanan yang dilakukan mengikuti standar mutu yang telah ditetapkan. QCC memiliki fokus yang lebih spesifik pada tindakan perbaikan di level operasional, sedangkan QCP lebih menekankan pada perencanaan, evaluasi, dan pengendalian kualitas di tingkat manajemen.
Conjunto de modelos QCP 2 slides de apresentação em Powerpoint Gráficos de apresentação
Struktural kegiatan Quality Control Circle adalah sebagai berikut : 1. Panitia pembimbing. Panitia pembimbing merupakan kelomok kerja yang diambil dari departemen QA (Quality Assurance). Mereka akan menjadi inisiator, perumus dan kebijakan untuk implementasi kegiatan QCC, seperti memutuskan usulan yang telah didiskusikan dalam kelompok Quality.
PPT FieldInduced Quantum Critical Point in CeCoIn 5 PowerPoint Presentation ID3658732
Training Improvement QCC/P dan SS. Penjelasan Umum. Agar perusahaan mampu bertahan dalam persaingan yang ketat dewasa ini, perusahaan dituntut bisa melakukan perbaikan dan inovasi berkelanjutan dalam proses bisnisnya. Banyak perusahaan tumbuh, namun kemudian gagal berkembang dikarenakan tidak bisa melakukan inovasi dalam bisnisnya.

QCP New Brand & Website Strategy Origo Branding Company
Quality Assurance vs Quality Control. Whereas quality assurance is focused on the processes involved in producing the output, quality control (QC) is more on the inspection of the quality of the products. This is performed by evaluating if they pass specific quality standards before being shipped out to customers.

Understanding the Different QCP Licenses AWI QCP
Di atas adalah salah satu makna QCP. Anda dapat mengunduh gambar di bawah ini untuk mencetak atau membagikannya kepada teman-teman Anda melalui Twitter, Facebook, Google atau Pinterest. Jika Anda seorang webmaster atau blogger, jangan ragu untuk memposting gambar di situs web Anda. QCP potrebbe avere altre definizioni.

What Licensing & Certification Mean in QCP’s Quality Assurance Process
Here is the best definition of Witness Point and Hold Point: Hold Point is a mandatory verification point beyond which work cannot proceed without approval by the Engineer or Consultant or Municipality Inspector. The work cannot proceed until the Engineer or Consultant is able to verify the quality of the completed work and releases the Hold by.

QCP From TikTok Was Reportedly Arrested — What Happened?
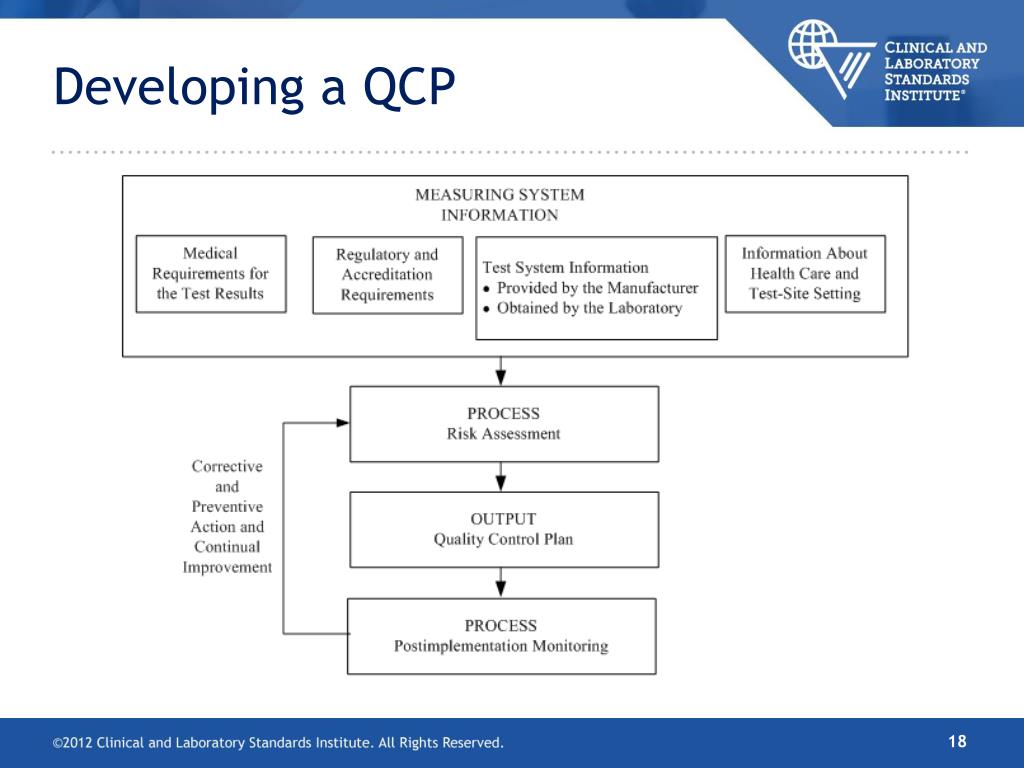
A strong, well-documented QCP will establish control procedures that reduce the likelihood of providing an inaccurate patient test result. Your QCP must at least include the number, type and frequency of testing and criteria for acceptable result(s) of the quality control(s). Your data must support the rationale for the number, type and
Quality Management System in Clinical Data Management
The QCP describes practices, resources, and procedures necessary to control the quality of a given test process. Using risk profiles, laboratories may determine that an individual test requires an individual QCP, or that tests with similar risk profiles may be grouped together in a single QCP. The QCP will also describe how to monitor.
Quality Assurance Program (QAP) HORIBA
1.3.2 Pengertian Quality Control Project (QCP) Quality Control Project (Jurnal Agrovaria, 2005) adalah Quality Control Circle yang melakukan suatu pemecahan masalah sebagai suatu project dimana anggotanya berkumpul secara sukarela dan berasal dari bidang kerja yang berbeda yang berkumpul dengan tujuan memecahkan masalah yang terjadi pada lintas departemen yang saling berhubungan

QCP File What is a .qcp file and how do I open it?
A CCP is defined as the most effective point in the process at which control, when applied, will prevent, eliminate, or reduce a food safety hazard to or below an acceptable level of safety, whether that is an FDA or USDA action level or the company's own food safety standard. A CQP is a step (point, procedure, operation, or stage) in food.

QCP Project Certification Explained AWIQCP
Guidance on conducting validation on CCP, QCP, PRP & oPRP - posted in FSSC 22000 Food Manufacturing: Hello, I want to know guidance in conducting validate the CCP, QCP, PRP & oPRP. It is monitored and checked on a daily basis as per set frequency but is required to validate. I am looking for your experience. Experts kindly share any template or relevant links.
Qcp 파일 문서 아이콘, 문서, 문서, 파일 PNG, 일러스트 및 벡터 에 대한 무료 다운로드 Pngtree
Quality control (QC) adalah proses penting yang wajib dilewati setiap perusahaan atau bisnis, terutama jika mereka memproduksi baik itu produk maupun jasa. QC ini harus dilewati dalam proses desain produk, software development, penjualan produk yang diproduksi secara massal, dan masih banyak lagi. Kalau tidak melalui proses ini, bisa jadi ada.

PPT T erminologi & Konsep Dasar Mutu , Jaminan dan Kendali Mutu Radiologi PowerPoint
An "Inspection and Test Plan" (ITP) might also be called a "Quality Inspection Plan". Inspection and Test Plans set out critical control points or 'hold points' at various stages within a process. Each control point is a scheduled inspection or verification activity where you will make sure that things are progressing as they should be, and get.
PPT CLSI EP23 ™ —Laboratory Quality Control Based on Risk Management PowerPoint Presentation
HACCP dalam pelaksanaannya terdiri atas beberapa tahapan. Di dalam tahapan tersebut terdapat dua pendekatan dalam pengendalian risiko, yaitu CCP dan OPRP. CCP (Critical Control Point) adalah suatu tindakan khusus yang dilakukan untuk menghilangkan bahaya spesifik. Caranya dengan menetapkan titik kritis bahaya sebagai acuan kendali.

The Quality Certification Program for Woodworkers AWI QCP
It is helpful to include the manufacturer's recommendations for QC to assure the QCP is not less than what is recommended. QCP must ensure accuracy and precision of the test/assay. The laboratory director (whose name should be on the CLIA license) must review and approve the QCP, sign and date it, and include the implementation date. 4. 3.