P9702287 — ChemDiv Screening compound NmethylN[(oxan4yl)methyl]4propylbenzene1sulfonamide
In this reaction three reactions are required. 1) A nitration. 2) A conversion from the nitro group to an amine. 3) A bromination. Because the end product is meta a meta directing group must be utilized. Of the nitro, bromine, and amine group, only the nitro group is meta direction. This means that the first step need to be the nitration and.
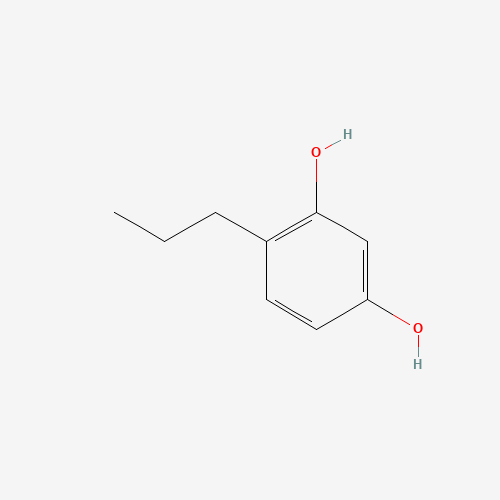
4propylbenzene1,3diol escientificsolutions
Other names: n-Propylbenzene; Isocumene; Propylbenzene; 1-Phenylpropane; 1-Propylbenzene; Phenylpropane; UN 2364; Benzene, n-propyl-; NSC 16941 Permanent link for this species. Use this link for bookmarking this species for future reference. Information on this page: Gas phase thermochemistry data; Condensed phase thermochemistry data; Phase.
HPLC Methods for analysis of Benzyl alcohol HELIX Chromatography
Alkylation of benzene with propene over acid catalysts yields isopropylbenzene (cumene) accompanied by formation of n-propylbenzene, di-isopropylbenzenes and propene oligomers as main by-products. The alkylation process proceeds via carbenium ions formed from olefins, which react with aromatic molecules generating the products [2].
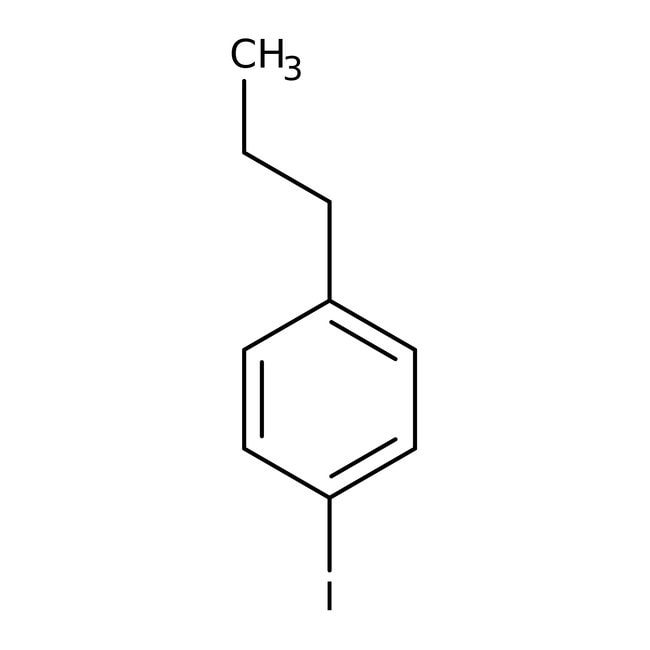
1Iodo4npropylbenzene, 97, Thermo Scientific™
Propylbenzene. Propylbenzene may refer to: n -Propylbenzene, the straight chain isomer (IUPAC name propylbenzene) Cumene (isopropylbenzene) This set index article lists chemical compounds articles associated with the same name. If an internal link led you here, you may wish to change the link to point directly to the intended article.

1Bromo4npropylbenzène, 99 , Thermo Scientific Chemicals
Other names: n-Propylbenzene; Isocumene; Propylbenzene; 1-Phenylpropane; 1-Propylbenzene; Phenylpropane; UN 2364; Benzene, n-propyl-; NSC 16941 Permanent link for this species. Use this link for bookmarking this species for future reference. Information on this page: Notes; Other data available: Gas phase thermochemistry data

2 Phenyl Propane Images, Stock Photos & Vectors Shutterstock
Reactions that occur at the benzylic position are very important for synthesis problems. So let's look at a few. We'll start with the free radical bromination of alkyl benzenes. And so here is my alkyl benzene, so a benzene ring, and I have an alkyl group attached to that. So this is a carbon.
D4006522 — ChemDiv Screening compound 3(5tertbutyl1,2,4oxadiazol3yl)4methoxyN
n-Propylbenzene is an aromatic hydrocarbon with the formula C 6 H 5 CH 2 CH 2 CH 3. The molecule consists of a propyl group attached to a phenyl ring. It is a colorless liquid. A more common structural isomer of this compound is cumene.

Propylbenzene, 98 103651 Manufacturers & Suppliers in India with worldwide shipping.
NIST/TRC Web Thermo Tables (WTT) NIST Standard Reference Subscription Database 3 - Professional Edition Version 2-2012-1-Pro This web application provides access to a collection of critically evaluated thermodynamic property data for pure compounds with a primary focus on organics. These data were generated through dynamic data analysis, as implemented in the NIST ThermoData Engine software.

1Nitro4npropylbenzene, 96, Thermo Scientific Fisher Scientific
Other names: n-Propylbenzene; Isocumene; Propylbenzene; 1-Phenylpropane; 1-Propylbenzene; Phenylpropane; UN 2364; Benzene, n-propyl-; NSC 16941 Permanent link for this species. Use this link for bookmarking this species for future reference. Information on this page: Mass spectrum (electron ionization) References; Notes; Other data available:
L2200314 — ChemDiv Screening compound N{[1(3methylbenzoyl)2,3dihydro1Hindol6yl]methyl
The role of different vibrational modes in the energy transfer from highly vibrationally excited CS2 is investigated in model classical trajectory calculations. The primary tool for this work involves determining the dependence of the average energy transfer on the vibrational frequency of each mode. These calculations show that the energy transfer is highly sensitive to the frequency of the.
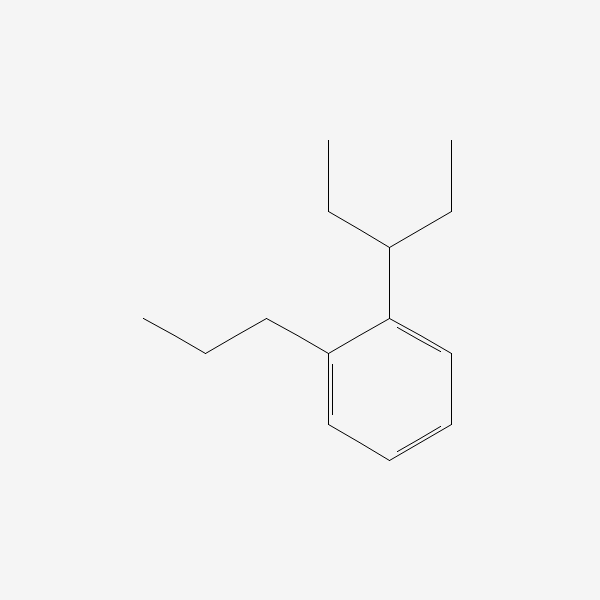
1(1ETHYLPROPYL)2PROPYLBENZENE
16.11: Synthesis of Polysubstituted Benzenes. As discussed in the Introduction to Organic Synthesis in Section 9.9, one of the surest ways to learn organic chemistry is to work synthesis problems. The ability to plan a successful multistep synthesis of a complex molecule requires a working knowledge of the uses and limitations of a great many.
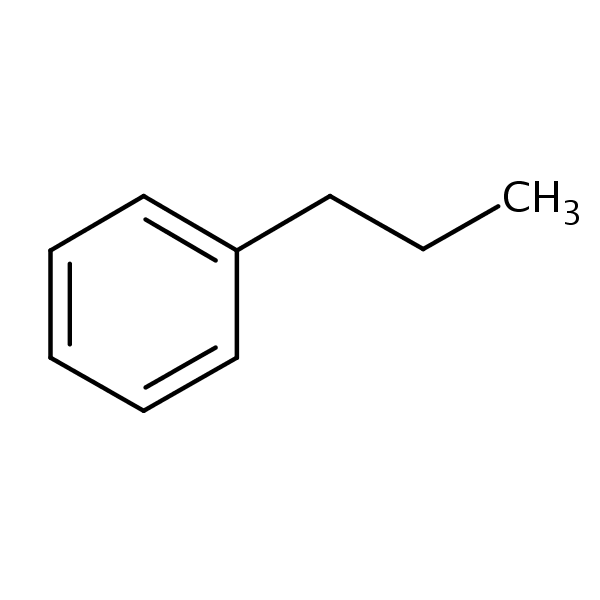
Propylbenzene SIELC Technologies
Propylbenzene contains an alkyl substituent. As in the case of a methyl group, the propyl group is slightly activating. Thus, propylbenzene is more reactive than benzene. Ethyl benzoate has a carbonyl carbon atom bonded directly to the aromatic ring. As a result, its rate of bromination will be significantly slower than that of benzene.
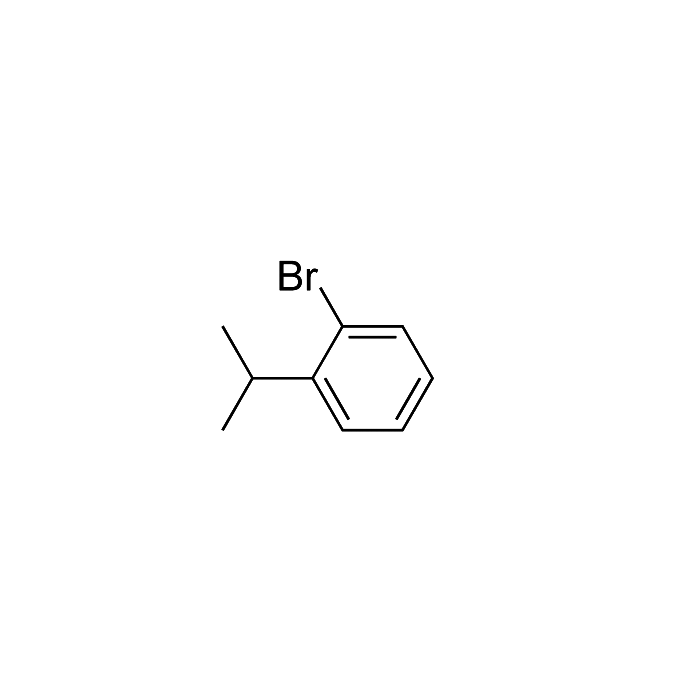
1Bromo2isopropylbenzene Kingchem
Other names: n-Propylbenzene; Isocumene; Propylbenzene; 1-Phenylpropane; 1-Propylbenzene; Phenylpropane; UN 2364; Benzene, n-propyl-; NSC 16941 Permanent link for this species. Use this link for bookmarking this species for future reference. Information on this page: Phase change data; References; Notes; Other data available: Gas phase.
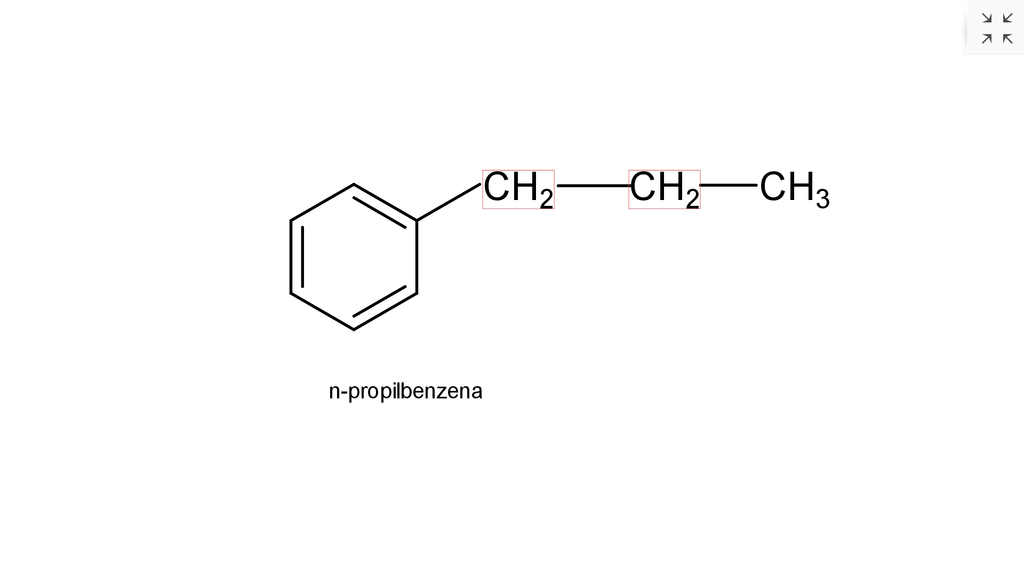
Tuliskan rumus struktur dari senyawa turunan benze...
Remarkably, a near-quantitative carbon yield was observed when using birch lignin, and the selectivity to arenes (methylbenzene M14, ethylbenzene M15, and propylbenzene M16) was as high as 71 wt %. The arenes were obtained by direct hydrodeoxygenation of organosolv lignin over a porous Ru/Nb 2 O 5 catalyst in water at 250 °C.
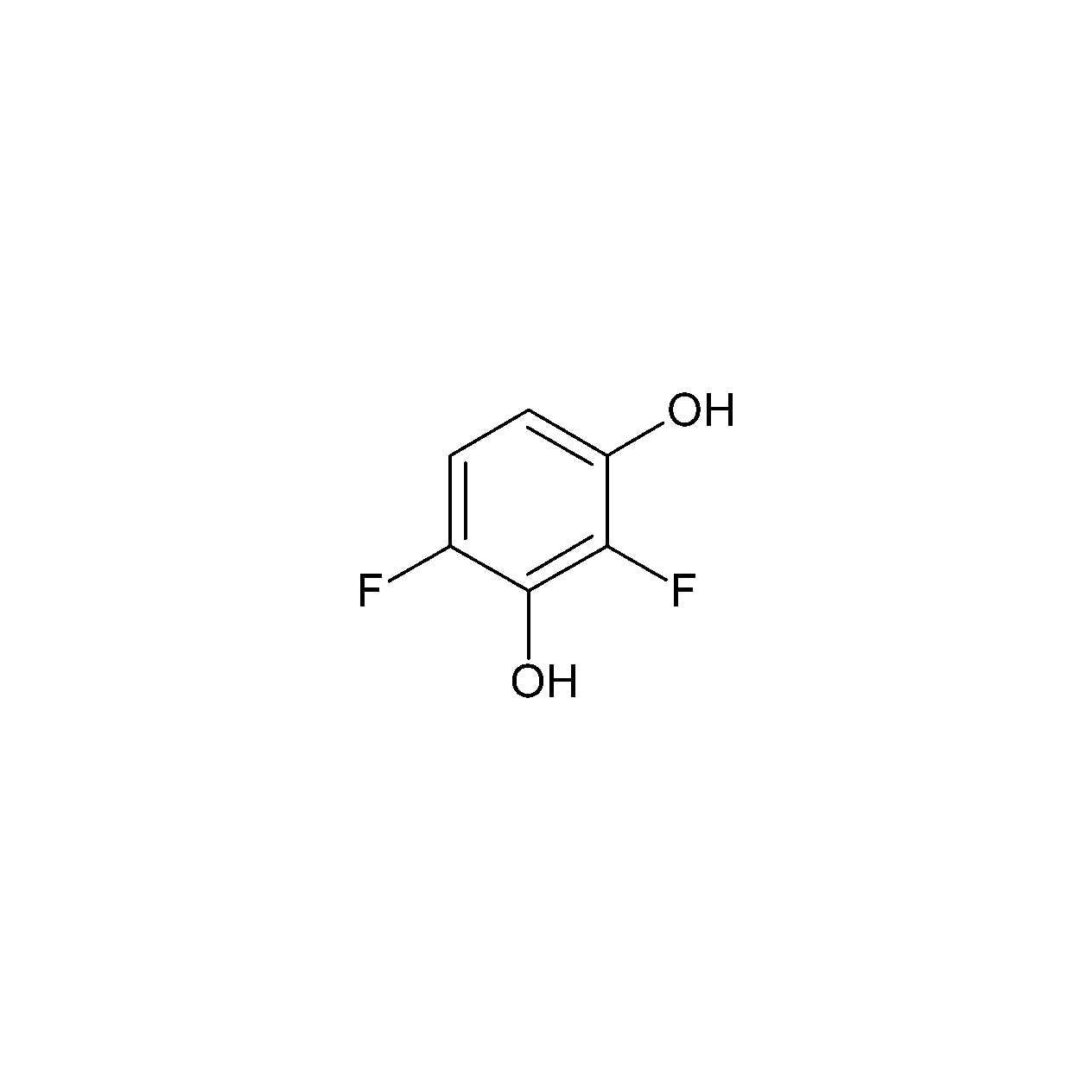
2,4Difluororesorcinol CASNumber 195136711 Order from Chemodex
The naming process for 2-chlorophenol (o-chlorophenol). Note that 2-chlorophenol = o-chlorophenol. Below is a list of commonly seen benzene-derived compounds. Some of these mono-substituted compounds (labeled in red and green), such as phenol or toluene, can be used in place of benzene for the chemical's base name.
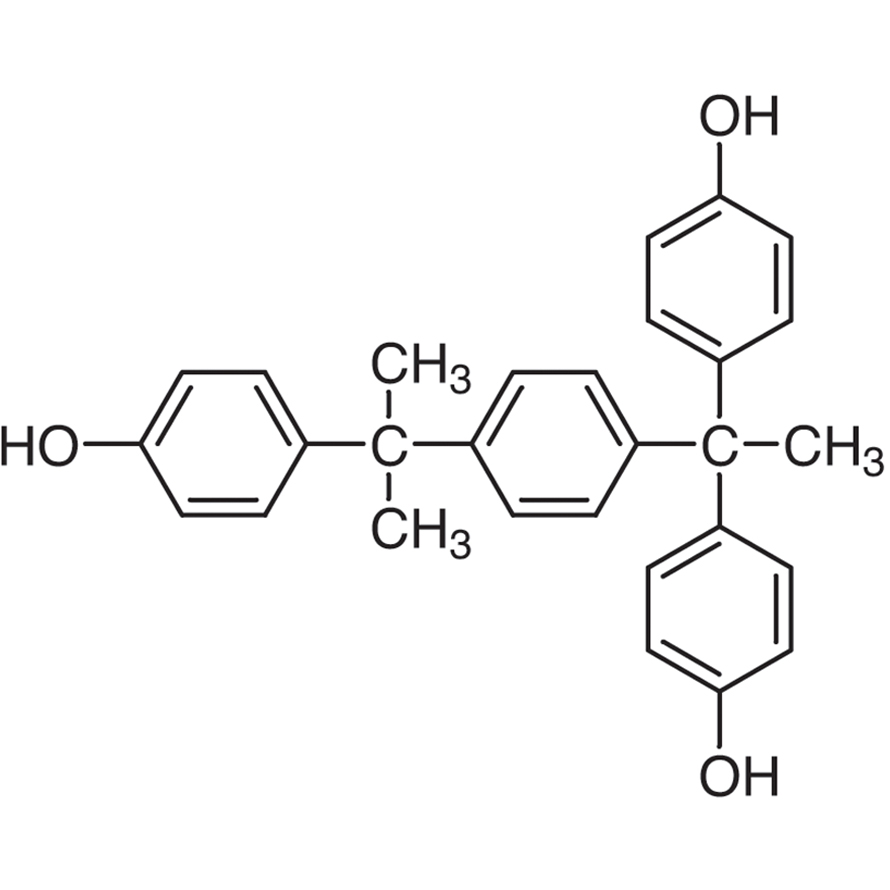
α,α,α'Tris(4hydroxyphenyl)1ethyl4isopropylbenzene 3BT1428
Propylbenzene | C9H12 | CID 7668 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety.