
Stable Quality Naso4 Filler Masterbatch for PE PE Plastic Production China Shrink Film Naso4
Sodium Sulfate Anhydrous is the anhydrous, sodium salt form of sulfuric acid. Sodium sulfate anhydrous disassociates in water to provide sodium ions and sulfate ions. Sodium ion is the principal cation of the extracellular fluid and plays a large part in the therapy of fluid and electrolyte disturbances.
NasoSafe Quest Surgical National
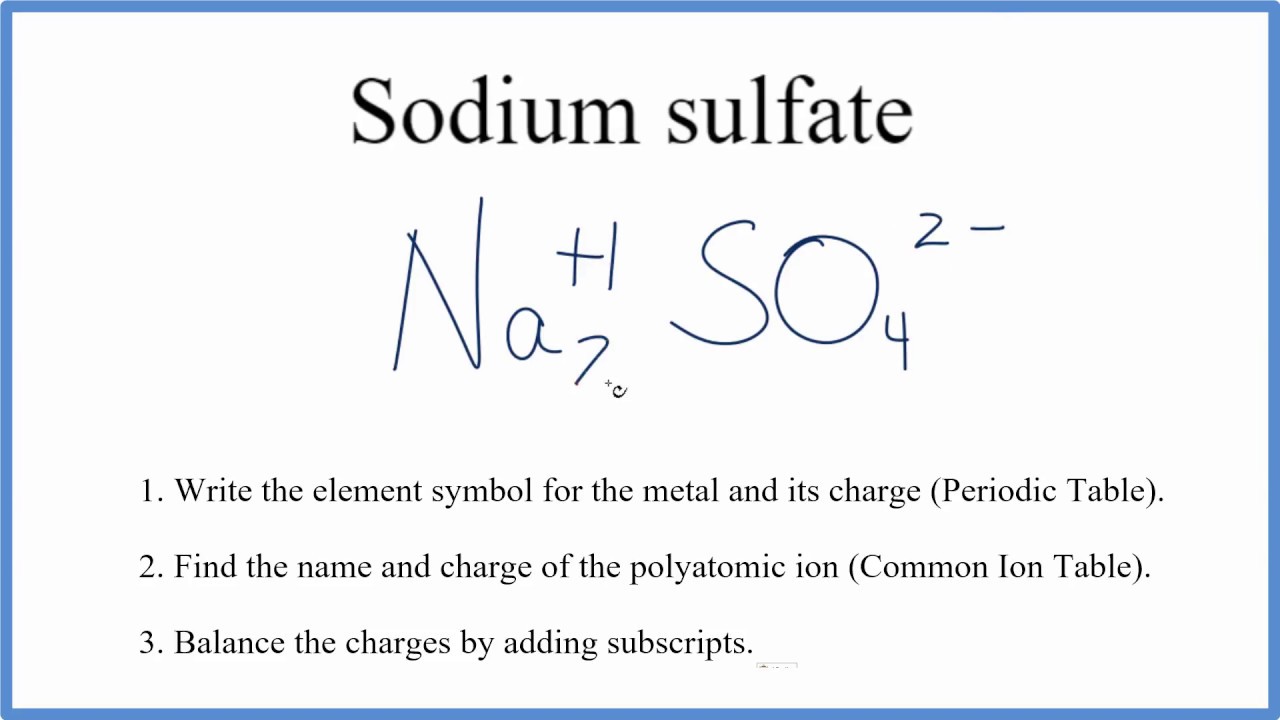
In this video we'll write the correct formula for Na2SO4 (Sodium sulfate).To write the formula for Na2SO4 we'll use the Periodic Table, a Common Ion Table, a.

China High Transparent NaSO4 Filler Masterbatch For PP/PE Suppliers and Manufacturers
Since there is an equal number of each element in the reactants and products of NaO + H2SO4 = NaSO4 + H2O, the equation is balanced. Balance NaO + H2SO4 = NaSO4 + H2O Using Inspection The law of conservation of mass states that matter cannot be created or destroyed, which means there must be the same number atoms at the end of a chemical.

Triethylamine hydrochloride
Learn about sodium sulfate, a white powder that is water-soluble and has various forms and applications. Find out how it is prepared, its history, and its structure with hydrogen bonds and octahedral lattice.

original drawn by naso4 Danbooru
Quantity Value Units Method Reference Comment; Δ f H° gas-1033.62: kJ/mol: Review: Chase, 1998: Data last reviewed in June, 1978: Quantity Value Units Method Reference Comment

The chemical structure of Poly (sodium 4 styrene sulfonate) Download Scientific Diagram
Step 4: Substitute Coefficients and Verify Result. Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. Since there is an equal number of each element in the reactants and products of NaSO4 = Na + SO4, the equation is balanced.

Why is it that when aluminium sulphate is dissolved in water, the solution acidic
Sodium Sulphate, NaSO4, is a white crystalline solid that is very soluble in water. It is produced industrially by the reaction of sulfur dioxide with sodium carbonate. Sodium carbonate is made by the calcination of sodium bicarbonate: 2 NaHCO3 → Na2CO3 + H2O + CO2. The carbon dioxide gas formed in this reaction is collected and used to.

How to Draw the Lewis Dot Structure for Na2SO4 Sodium sulfate YouTube
Sodium bisulfate, also known as sodium hydrogen sulfate, is the sodium salt of the bisulfate anion, with the molecular formula NaHSO 4.Sodium bisulfate is an acid salt formed by partial neutralization of sulfuric acid by an equivalent of sodium base, typically in the form of either sodium hydroxide (lye) or sodium chloride (table salt). It is a dry granular product that can be safely shipped.

CaCO3, Naso4, Baso4 Filler Masterbatches for Plastic Films China Masterbatch and Filler
To find the mole fraction and percentage of each element in NaSO4, divide each total from step 3 by the total molar mass found in step 4: Mole Fraction Percent; Na (Sodium) 22.98976928 ÷ 119.05236928 = 0.193106: × 100 = 19.3106%: S (Sulphur/Sulfur) 32.065 ÷ 119.05236928 = 0.269335:
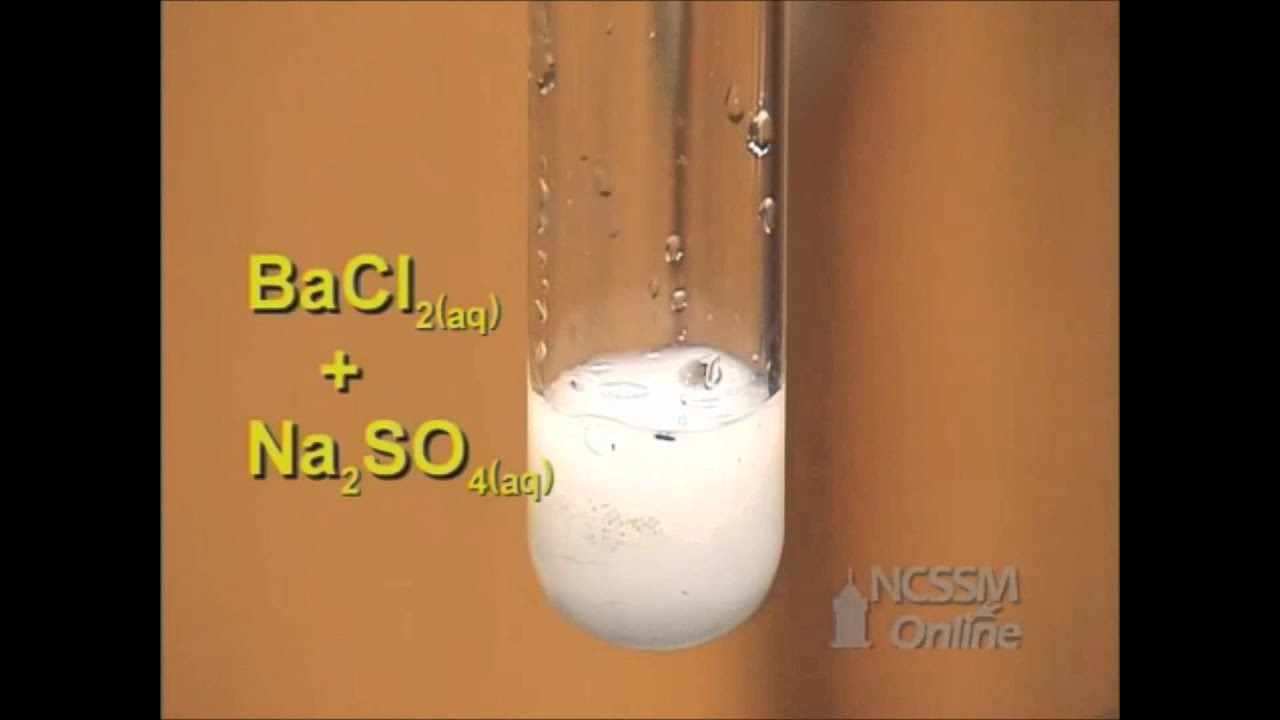
NaCl H2SO4
Notice: Except where noted, spectra from this collection were measured on dispersive instruments, often in carefully selected solvents, and hence may differ in detail from measurements on FTIR instruments or in other chemical environments. More information on the manner in which spectra in this collection were collected can be found here. Notice: Concentration information is not available for.

Hóa Chất Sodium Molybdate AR
NaSO4 molecular weight. Molar mass of NaSO4 = 119.05237 g/mol. Convert grams NaSO4 to moles. or. moles NaSO4 to grams. Molecular weight calculation: 22.98977 + 32.065 + 15.9994*4. Percent composition by element. Element: Sodium Symbol: Na Atomic Mass: 22.989770 # of Atoms: 1 Mass Percent: 19.311%. Element: Oxygen

glasses naso4 orange eyes red hair scoobydoo short hair velma dace dinkley white
J/ (mol·K) Solid properties. Std enthalpy change. of formation, Δ fH o solid. -1387.1 kJ/mol. Standard molar entropy, S o solid. 149.6 J/ (mol K) Heat capacity, cp.

Sodium Sulfat / NaSO4 SIL
4. 53.7558. Computing molar mass step by step. First, compute the number of each atom in NaSO 4: Na: 1, S: 1, O: 4. Then, lookup atomic weights for each element in periodic table: Na: 22.98976928, S: 32.065, O: 15.9994. Now, compute the sum of products of number of atoms to the atomic weight: Molar mass (NaSO 4) = ∑ Count i * Weight i =.
So4 Dot And Cross Diagram
To tell if Na2SO4 (Sodium sulfate) forms an acidic, basic (alkaline), or neutral solution we can use these three simple rules along with the neutralization r.
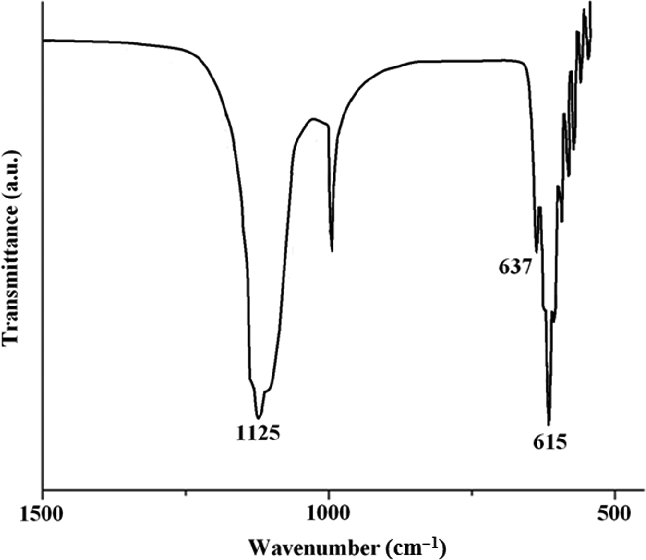
FTIR spectrum of Na 2 SO 4 nanorods. Download Scientific Diagram
Sodium sulphate (Na2SO4) is the sodium salt of sulphuric acid. It is a white crystalline solid with no water in its anhydrous form and a natural mineral form called Glauber's salt. It is used as a drying material, a dehydrating agent, a laxative, and in various consumer products. Learn more about its structure, properties, preparation, uses and FAQs.
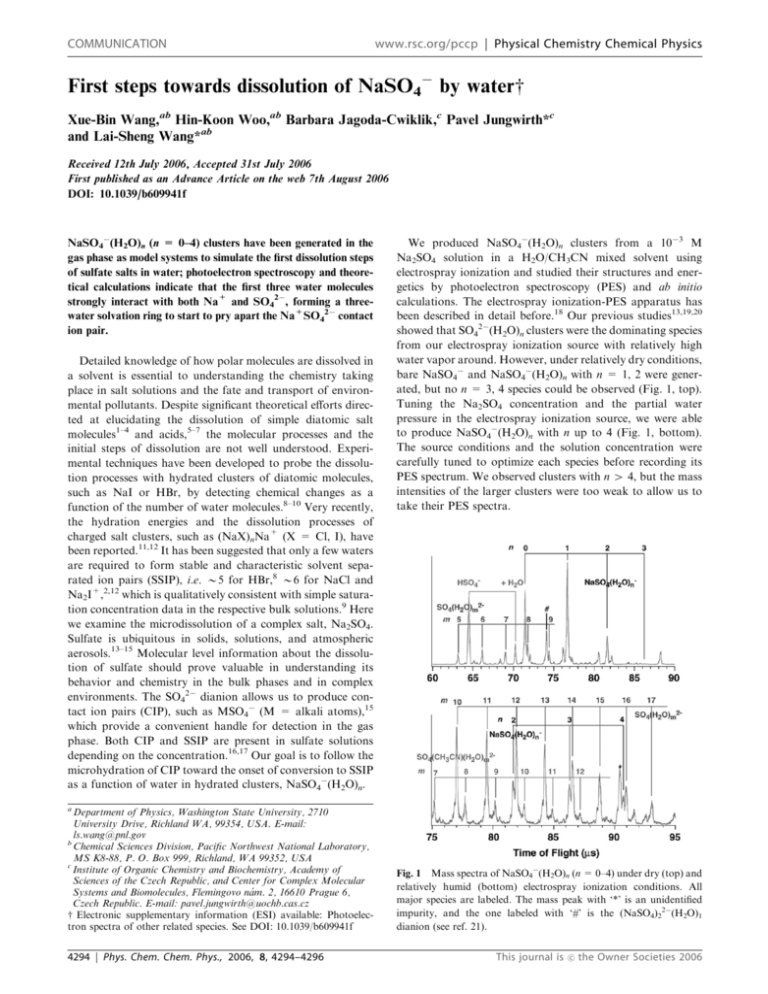
First steps towards dissolution of NaSO4 by waterw
Sodium sulfate (also known as sodium sulphate or sulfate of soda) is the inorganic compound with formula Na 2 SO 4 as well as several related hydrates.All forms are white solids that are highly soluble in water. With an annual production of 6 million tonnes, the decahydrate is a major commodity chemical product. It is mainly used as a filler in the manufacture of powdered home laundry.