
PPT Geometric ISOMERS of Alkenes PowerPoint Presentation, free download ID2372292
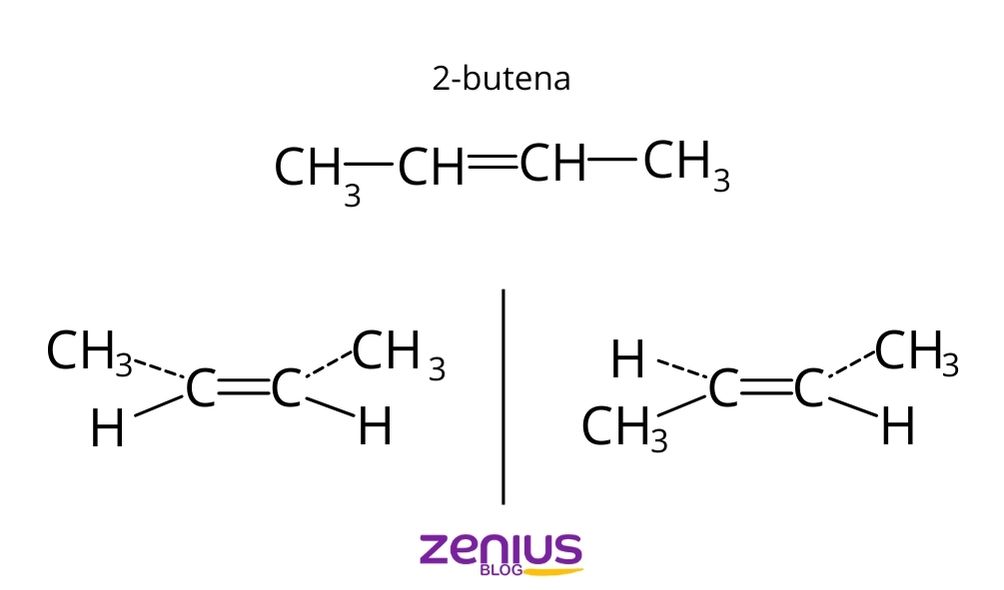
Kedua atom metil di cis-2 butena terkait karbon. Lalu tetap berada pada satu sisi ruang geometris. Sedangkan pada trans-2 butena pada sisi berbeda dan bersebrangan. Penamaannya juga berbeda. Contoh Isomer Geometri Senyawa Misalnya isomer geometri senyawanya alkena pentana C5H10 pada 2-pentana. Isomer tersebut dari 2 pentana rumus kimianya C5H10.

Organic Molecules and Isomers Biology 201 The Chemistry of Life
Geometric Isomers of Alkenes. In the discussions about 1,2-dimethylcyclohexane in Chapter 4, we have learned that there are two geometric isomers possible for that compound, that are cis and trans.The restricted C-C bond rotation of cyclic structure result in the cis or trans isomer of 1,2-dimethylcyclohexane. Restricted rotation also can be caused by a double bond, so geometric isomers apply.
Six types of isomer structure Adobe Stock
cis-but-2-ene trans-but-2-ene. Cis-trans isomerism, also known as geometric isomerism, describes certain arrangements of atoms within molecules.The prefixes "cis" and "trans" are from Latin: "this side of" and "the other side of", respectively.In the context of chemistry, cis indicates that the functional groups (substituents) are on the same side of some plane, while trans conveys that they.

Geometric Isomers (9/11) Organic Chemistry NCEA Level 2 Chemistry StudyTime NZ YouTube
Tutorial illustrating geometric isomers in polymer repeat units. Illustrated with 3D model.Video lecture for Introduction to Materials Science & Engineering.

PPT Geometric ISOMERS of Alkenes PowerPoint Presentation, free download ID2372292
Geometric (cis / trans) isomerism. These isomers occur where you have restricted rotation somewhere in a molecule. At an introductory level in organic chemistry, examples usually just involve the carbon-carbon double bond - and that's what this page will concentrate on. Think about what happens in molecules where there is un restricted rotation.
Isomer Ruang dan Isomer Geometri Materi Kimia Kelas 12
Conformational Isomers. The C-C single bonds in ethane, propane, and other alkanes are formed by the overlap of an sp 3 hybrid orbital on one carbon atom with an sp 3 hybrid orbital on another carbon atom, forming a σ bond. Each sp 3 hybrid orbital is cylindrically symmetrical (all cross-sections are circles), resulting in a carbon-carbon single bond that is also cylindrically symmetrical.
Isomer Definition, Types, Example and Quiz Biology Dictionary
Isomers are molecules that have the same molecular formula, but have a different arrangement of the atoms in space. Consider butane: There are also endless other possible ways that this molecule could twist itself. There is almost completely free rotation around all the carbon-carbon single bonds. If you had a model of a molecule in front of.
24.2 Isomers of Organic Compounds Chemwiki
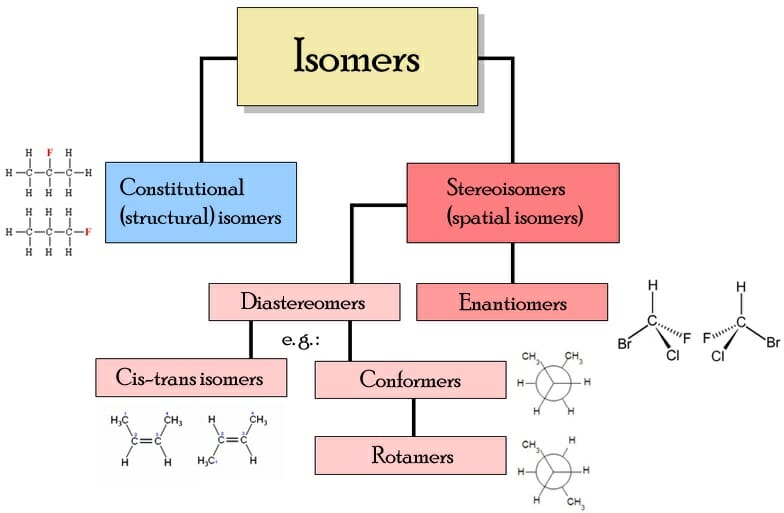
Isomer. In chemistry, isomers are molecules or polyatomic ions with identical molecular formula - that is, same number of atoms of each element - but distinct arrangements of atoms in space. [1] Diamond and graphite are a familiar example; they are isomers of carbon. Isomerism refers to the existence or possibility of isomers.
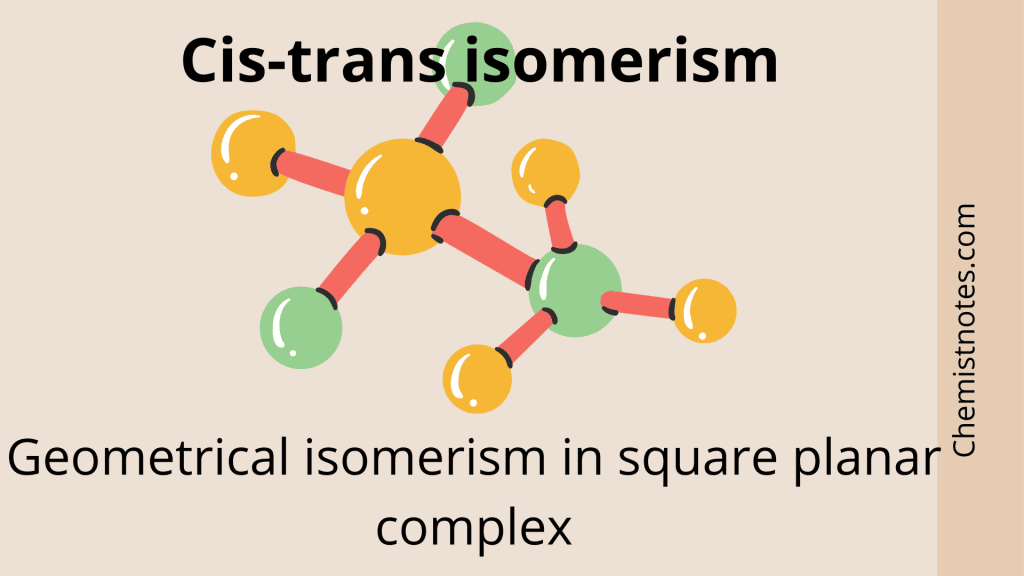
Geometrical Isomerism Cistrans isomerism Chemistry Notes
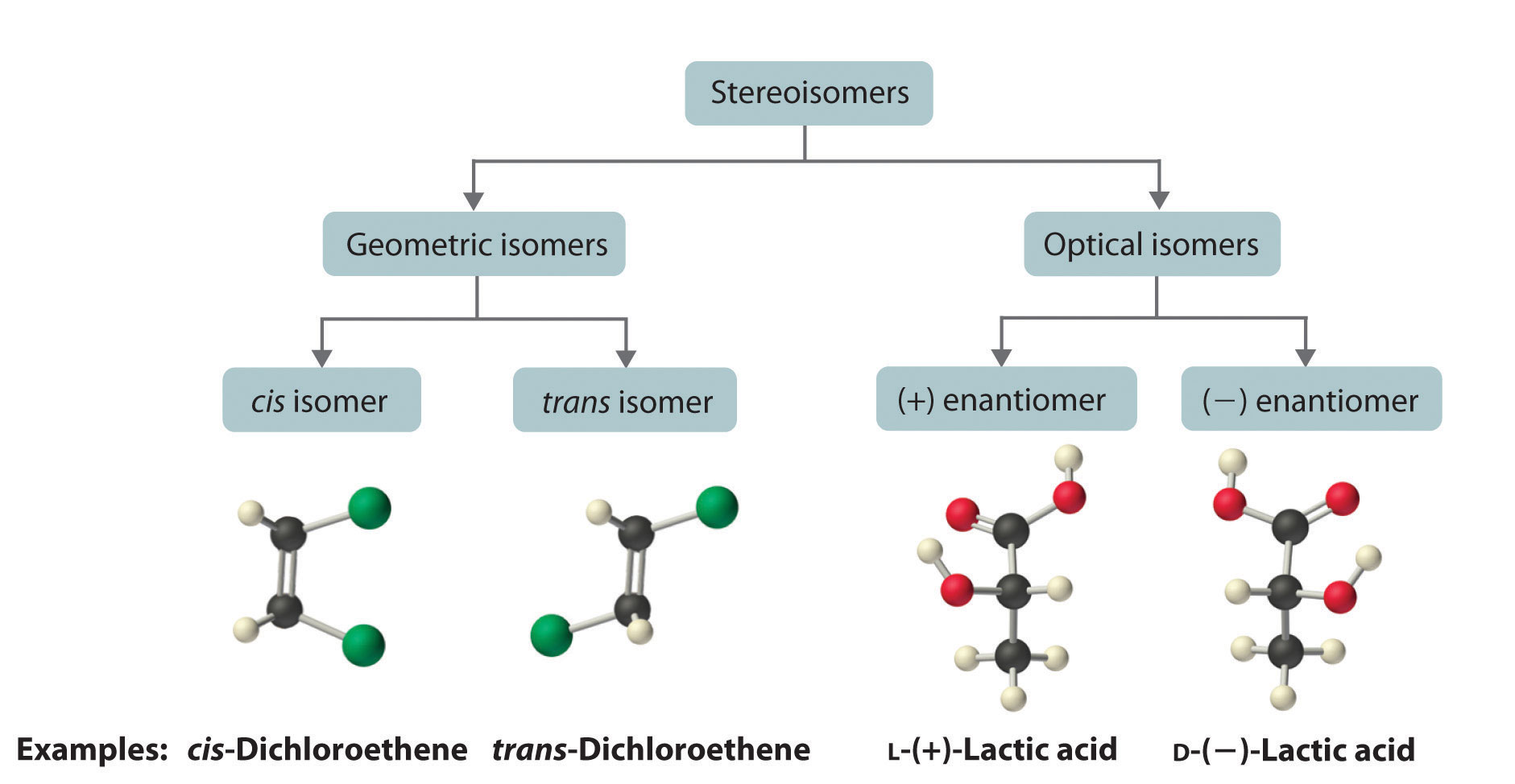
Geometric Isomers Definition. Geometric isomerism is a kind of stereoisomerism. It is also known as cis-trans isomerism or E-Z isomerism. Geometric isomerism occurs due to the restricted rotation about carbon-carbon double bonds or carbon-carbon single bonds in cyclic compounds. Geometric isomers are the stereoisomers which differ from each.
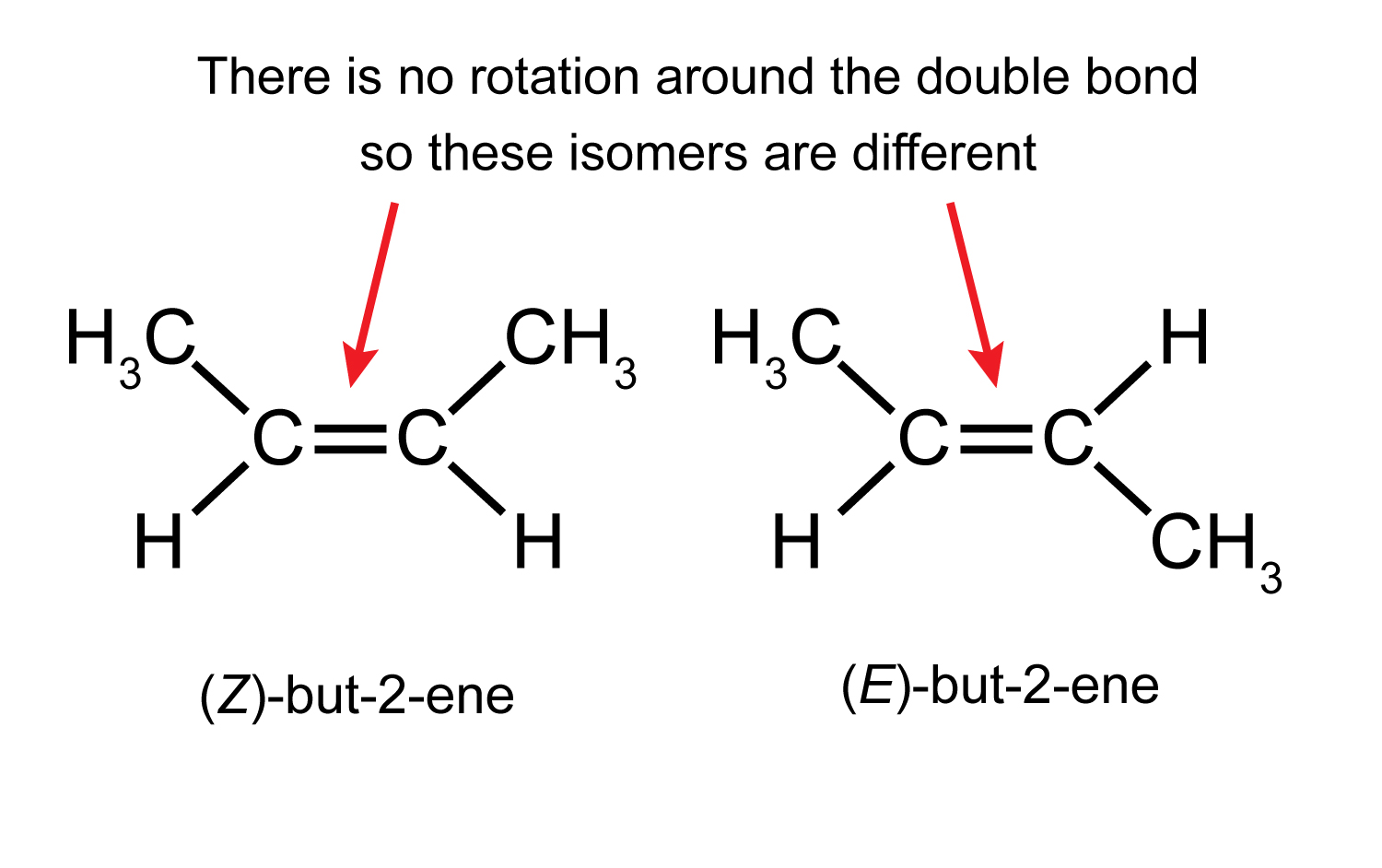
Maths skills Geometry Exploring Understanding Isomers
Geometric isomerism (also known as cis-trans isomerism or E-Z isomerism) is a form of stereoisomerism. This page explains what stereoisomers are and how you recognise the possibility of geometric isomers in a molecule. Further down the page, you will find a link to a second page which describes the E-Z notation for naming geometric isomers.
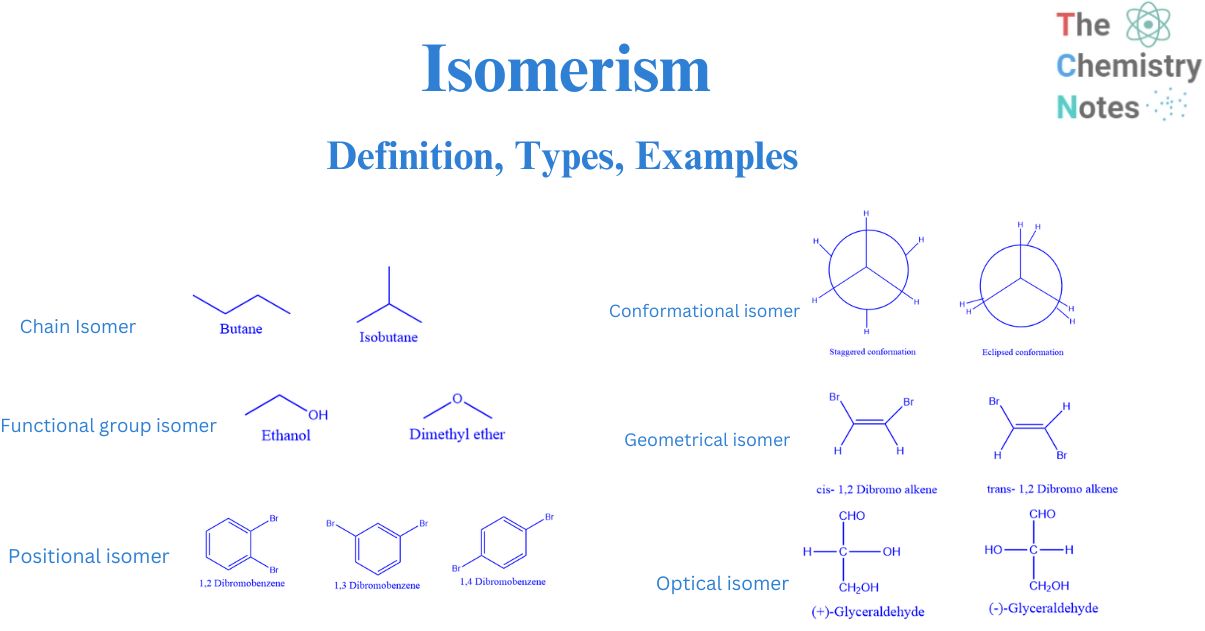
Isomerism Definition, Types, Examples
Cis-trans (geometric) isomerism exists when there is restricted rotation in a molecule and there are two nonidentical groups on each doubly bonded carbon atom. The IUPAC naming is the same as alkene except for the addition of the cis or trans prefix. 2.2: Geometric Isomers is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and.
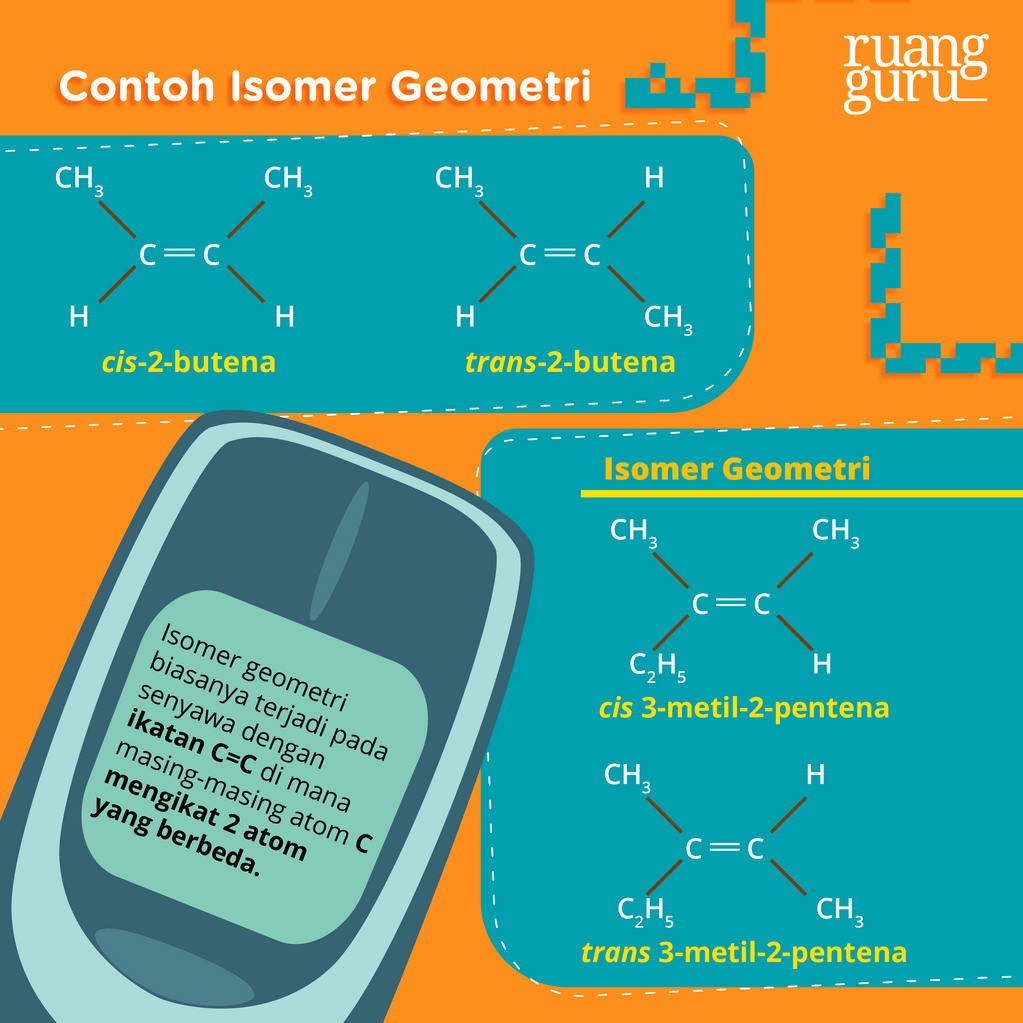
Kimia Kelas 11 Pengertian Isomer, Jenisjenisnya, Serta Contohnya Belajar Gratis di Rumah
Geometric isomers differ in the spatial arrangement of substituents on a double bond or non-aromatic ring system. Especially in the case of 1,2-disubstituted alkenes, one usually speaks of a cis-/trans- isomerism.In the case of longer chains and/or higher substituted compounds, naming according to the (Z)-, (E)- notation using the CIP rules is necessary to clearly indicate the configuration.

Optimized geometric structure of the Y(L)3 (a) Isomer 1 (more stable),... Download Scientific
Generally the number of isomers increases. You can demonstrate this to yourself by drawing all possible structures for propane (1), butanes (2), pentanes (3), and hexanes (5). One way to think about this is as follows: Each carbon you add can attach to any of the carbons already present in any isomer of the molecule.
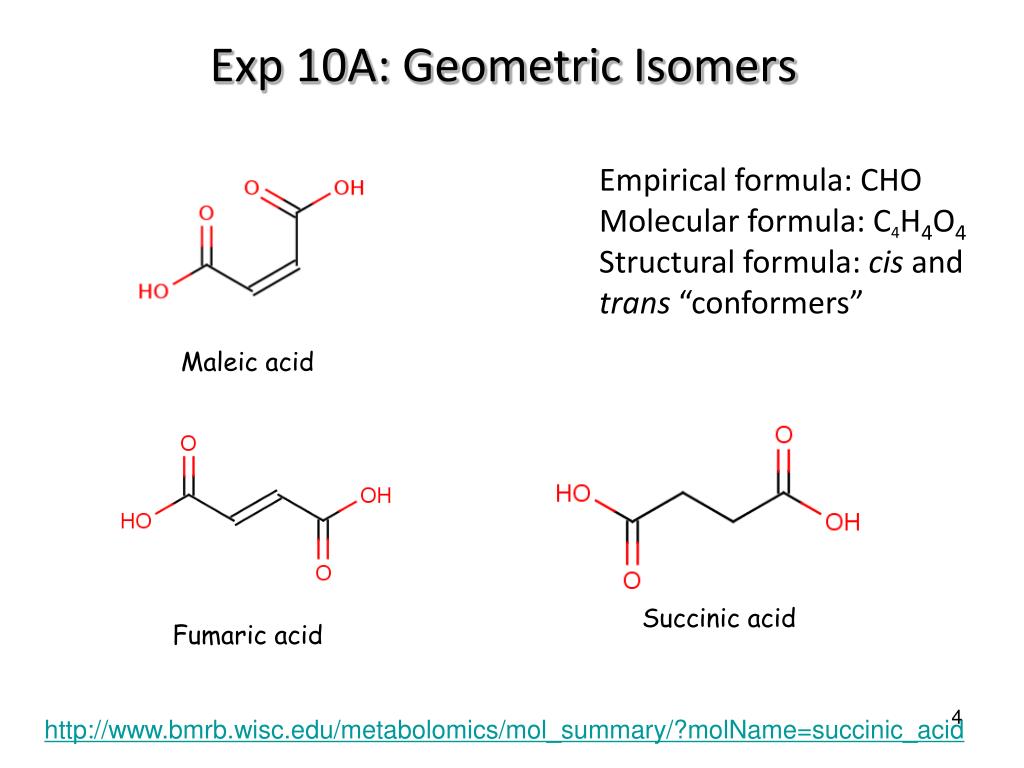
PPT Exp 10A Geometric Isomers PowerPoint Presentation, free download ID4494363
Although geometric isomers have completely different physical and chemical properties (for example, cis-and trans-2-butene have different boiling points and densities), optical isomers (also called enantiomers) differ in only one characteristic--their interaction with plane polarized light.When a beam of light is passed through a certain type of filter, all of the waves except those in one.
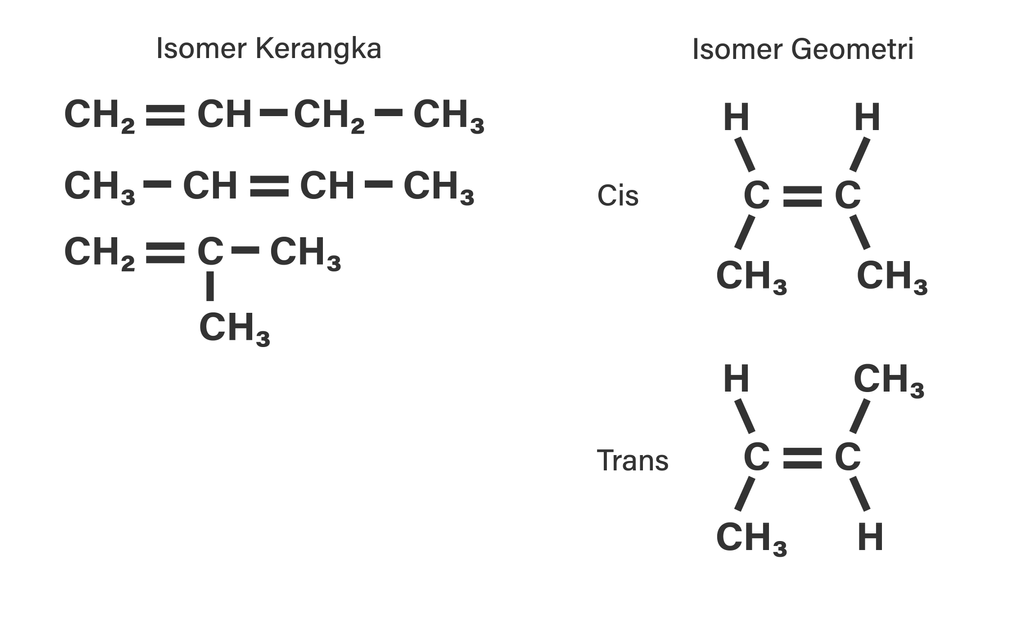
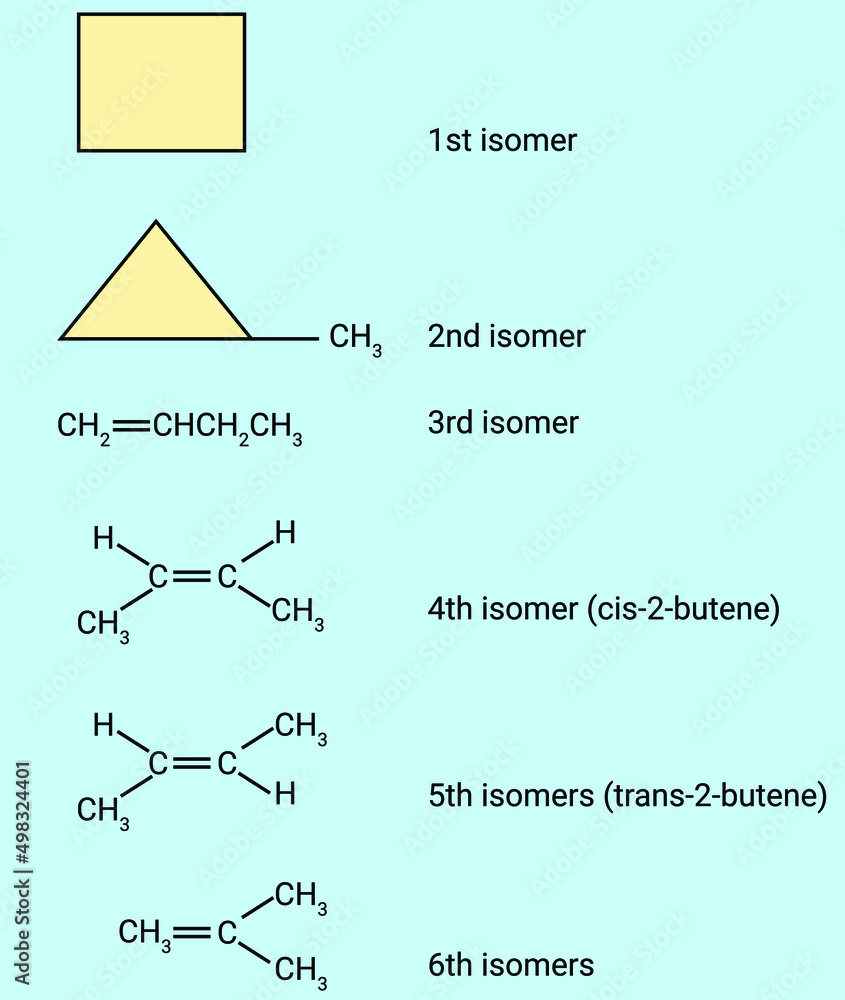
Tentukan isomerisomer dari C4H8 dengan menggambar...
Geometric isomers are chemical species with the same type and quantity of atoms as one another, yet having different geometric structures. In geometric isomers, atoms or groups exhibit different spatial arrangements on either side of a chemical bond or ring structure. Geometric isomerism is also called configurational isomerism or cis-trans.
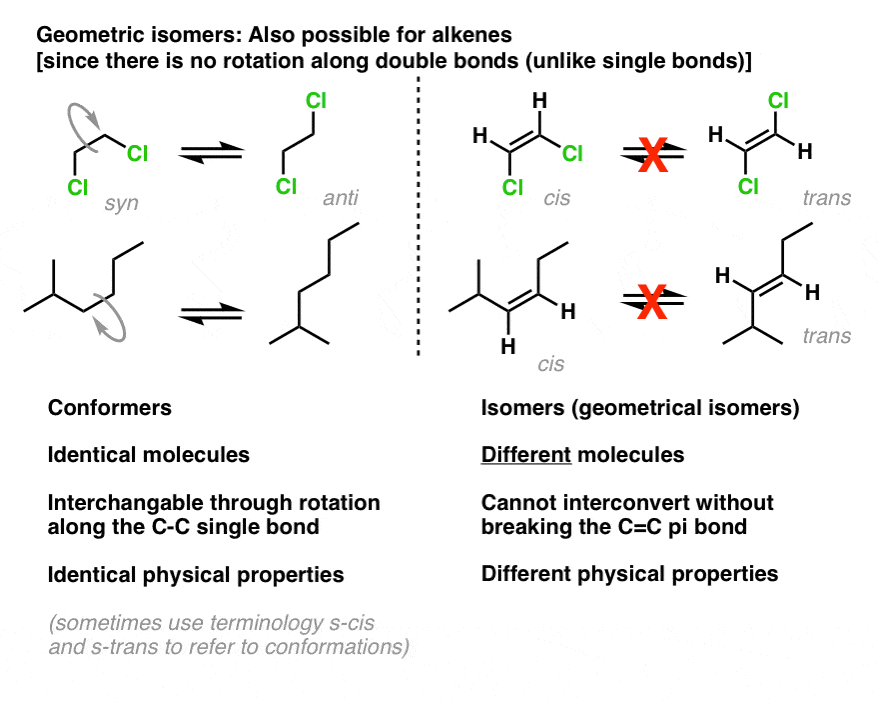
isomere geometrique definition
Geometric isomerism, also known as cis-trans isomerism, is a form of stereoisomerism. Like all stereoisomers, geometric isomers are compounds that are made up of the same constituent atoms and are connected in the same sequence but differ in the orientation of those atoms in space. All geometric isomers require restricted rotation within the.