
Hướng dẫn cách kclo3 cân bằng trong phản ứng hóa học hiệu quả nhất
Since there is an equal number of each element in the reactants and products of KClO + O2 = KClO3, the equation is balanced. Balance KClO + O2 = KClO3 Using Inspection The law of conservation of mass states that matter cannot be created or destroyed, which means there must be the same number atoms at the end of a chemical reaction as at the.
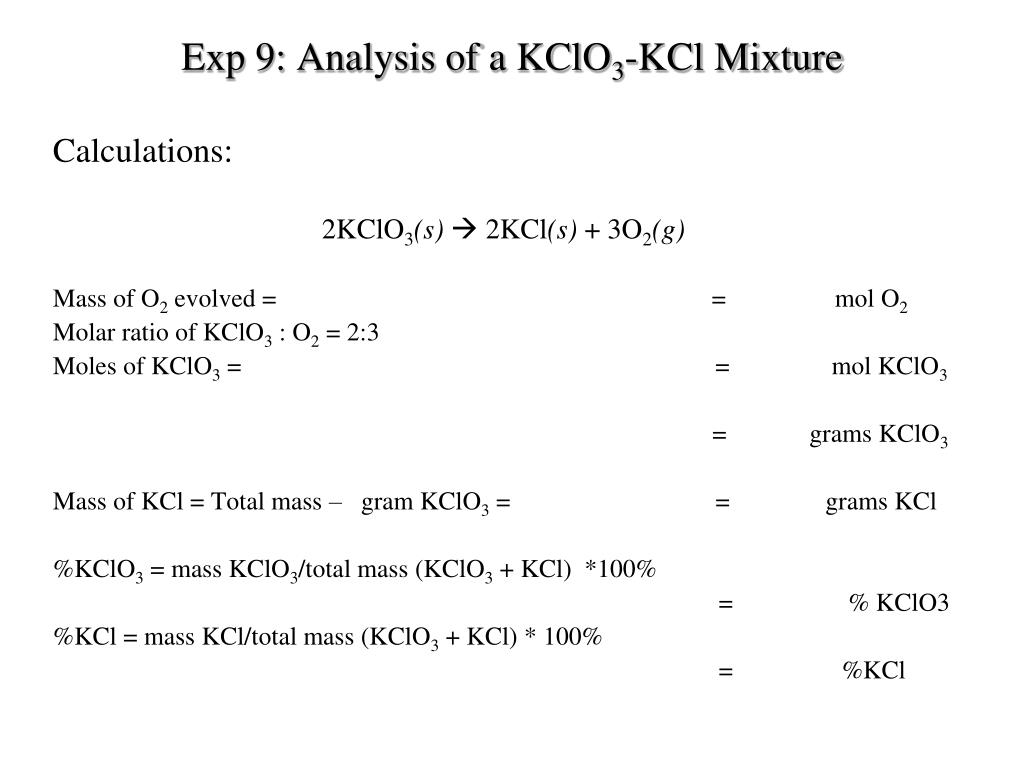
PPT Exp 9 Analysis of a KClO 3 KCl Mixture PowerPoint Presentation ID5084984
Mass Percent of Oxygen (experimental) = Mass of Oxygen Released Mass of Potassium Chlorate Used × 100 (5.3) (5.3) Mass Percent of Oxygen (experimental) = Mass of Oxygen Released Mass of Potassium Chlorate Used × 100. Mass percentages of elements in compounds can also be theoretically calculated using molar masses, along with the known.

Cara Membuat KClO3 Dengan Metode Klorinasi Elektrokimia (Elektrolisis) Bisakimia
Step 4: Substitute Coefficients and Verify Result. Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. 2 KCLO3 = 2 KCL + 3 O2. Reactants.

How to balance KClO3 → KCl + O2 YouTube
Step 4: Substitute Coefficients and Verify Result. Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. 2 KCl + 3 O2 = 2 KClO3. Reactants.

KCLO3>KCL+O2
3 + = 3 + KClO3. By formula: 3 C 5 H 5 NO + ClK = 3 C 5 H 5 N + KClO3. Quantity. Value. Units. Method. Reference. Comment. Δ r H°.

Figure 3 from Pressure induced structural phase transition in solid oxidizer KClO3 a first
Potassium chlorate (KClO 3) is a strong oxidizing agent that has a wide variety of uses. It is or has been a component of explosives, fireworks, safety matches, and disinfectants. As a high school or college chemistry student, you may have used it to generate oxygen in the lab. Because it is a strong oxidizer, KClO 3 must be kept from.

Molécula Kclo3 De Clorato Potásico. Fórmula Química Estructural Ilustración del Vector
Explanation: Start with the unbalanced equation: KClO3 → KCl +O2. A method that often works is to balance everything other than H and O first, then balance O, and finally balance H. Another rule is to start with what looks like the most complicated formula. The most complicated formula looks like KClO3. We put a 1 in front of it and mark the.
Cara Membuat KClO3 Dengan Metode Klorinasi Elektrokimia (Elektrolisis) Bisakimia
3 + = 3 + KClO3. By formula: 3 C 5 H 5 NO + ClK = 3 C 5 H 5 N + KClO3. Quantity. Value. Units. Method. Reference. Comment. Δ r H°.

Mno2 Kclo3 Koh Kmno4 Kcl H2o EDUCA
KClO3 molecular weight. Molar mass of KClO3 = 122.5495 g/mol. This compound is also known as Potassium Chlorate. Convert grams KClO3 to moles. or. moles KClO3 to grams. Molecular weight calculation: 39.0983 + 35.453 + 15.9994*3. Percent composition by element. Element: Chlorine Symbol: Cl Atomic Mass: 35.453

KCLO3 Kali clorat Potassium Chlorate HCTH HÓA CHẤT THANH HÓA™ TOP1
3. Compute Mass of Each Element. Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass of each element in KClO3: Molar Mass (g/mol) K (Potassium) 1 × 39.0983 = 39.0983. Cl (Chlorine) 1 × 35.453 = 35.453.

kclo3 ra o2 Phương trình phản ứng hóa học KClO3 → KCl + O2
2024-02-28. Description. Potassium chlorate appears as a white crystalline solid. Forms a very flammable mixture with combustible materials. Mixture may be explosive if combustible material is very finely divided. Mixture may be ignited by friction. Contact with strong sulfuric acid may cause fires or explosions.

La de KClO3 (s) en KCl (s) y O2 (g) con un rendimiento del 85. ¿Cuántos moles de
More information from the unit converter. How many moles KClO3 in 1 grams? The answer is 0.0081599680129254. We assume you are converting between moles KClO3 and gram.You can view more details on each measurement unit: molecular weight of KClO3 or grams This compound is also known as Potassium Chlorate.The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles KClO3, or.

kclo3 ra o2 Phương trình phản ứng hóa học KClO3 → KCl + O2
What is Potassium Chlorate (KClO3) - Potassium Chlorate is the most important salt of chloric acid, which is made by passing an excess of chlorine through a hot solution of potassium hydróxide or by heating a solution containing hypochlorite ion and potassium ion.
[Solved] Hi, can you help me to draw KClO3, KClO2, and KClO as a lewis... Course Hero
Since there is an equal number of each element in the reactants and products of KClO3 = K + ClO3, the equation is balanced. Balance KClO3 = K + ClO3 Using Inspection The law of conservation of mass states that matter cannot be created or destroyed, which means there must be the same number atoms at the end of a chemical reaction as at the.

How to Balance KClO3 = KCl + O2 of Potassium Chlorate) YouTube
The catalytic decomposition of KClO3 is studied in the presence of MnO2, activated carbons and sodium sulphide. This investigation describes the use of thermogravimetry as a convenient technique to investigate catalytic activity of a simple chemical reaction. It is observed that the MnO2 catalyst decreases the decomposition temperature from 480 to 350 °C while the activated carbons cause its.
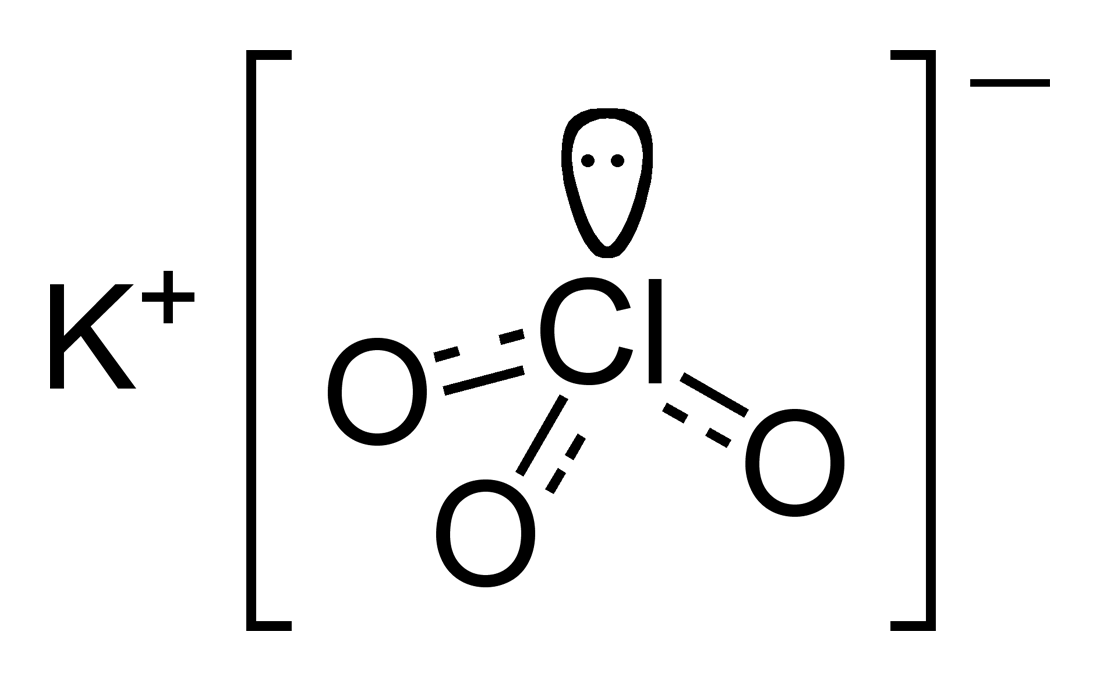
KClO3
Potassium chlorate is a compound containing potassium, chlorine and oxygen, with the molecular formula KClO 3.In its pure form, it is a white crystalline substance. After sodium chlorate, it is the second most common chlorate in industrial use. It is a strong oxidizing agent and its most important application is in safety matches. In other applications it is mostly obsolete and has been.