
Sf6 Polar Atau Nonpolar Brain
Chemistry Chemistry questions and answers Draw the lewis structure for ICl3 (iodine trichloride). What is the electron geometry and molecular geometry (shape) of the molecule? Refer to the provided chart to aid you in your answer. Is the molecule polar or non polar? This problem has been solved!

The Best 29 Icl3 Polar Or Nonpolar imageyardask
The chemical name of the $ IC {l_3} $ molecule is Iodine trichloride. It is an interhalogen compound made up of iodine and chlorine. Iodine has seven electrons and each chlorine provides one electron giving a total of five pairs.

Is Icl3 Polar Or Nonpolar
ICl3, named Iodine Trichloride, is an Interhalogen compound. Interhalogen compounds are molecules, which contain at least two different halogen atoms. These compounds are generally written as ABn where n= 1, 3, 5, and 7; A and B are less electronegative and more electronegative elements, respectively.

ICl3 Lewis, VSEPR, Hybridization, Energy Diagram, Contour Diagram YouTube
An explanation of the molecular geometry for the ICl3 (Iodine trichloride) including a description of the ICl3 bond angles. The electron geometry for the Iod.

VSEPR Theory Iodine Trichloride (ICl3) Expanded Valence YouTube
1 Answer. The bonding situation in the compound (IClX3)X2 ( I C l X 3) X 2 is by far more complex than what is depicted in this book. The molecule itself has very high symmetry, i.e. D2h D 2 h, that needs to be satisfied in the molecular orbital/ valence bond description. [1,2] That is why one cannot distinguish between the bridging chlorine.
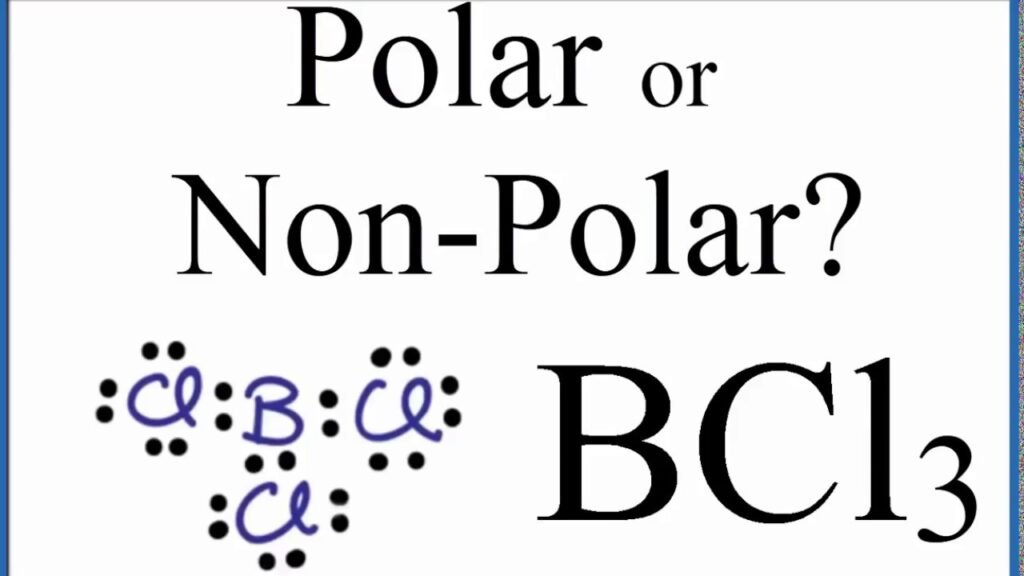
Is BCl3 Polar or Nonpolar?
ICl3 molecule is made of one iodine, three chlorine atoms. The chlorine and iodine atoms have s and p orbitals. Chlorine comes as the second element from the halogen family in the periodic table. The iodine atom also belongs to the same family group. But it falls as the fourth element in the periodic table.

Best Explanation CH2Cl2 polar or nonpolar [N01] Science Education
Explain how a molecule that contains polar bonds can be nonpolar. Answer. As long as the polar bonds are compensated (for example. two identical atoms are found directly across the central atom from one another), the molecule can be nonpolar. PROBLEM \(\PageIndex{2}\)

Determine whether CHCl3 is polar or nonpolar YouTube
March 25, 2023 in Science 0 ICL3 | Bond Angle, Molecular Geometry & Hybridization | Polar or Non Polar Iodine Trichloride Iodine trichloride or ICl3 is a chemical compound having an equation ICl3. It is a dark brown or red-orange solid that is extremely reactive and harmful.
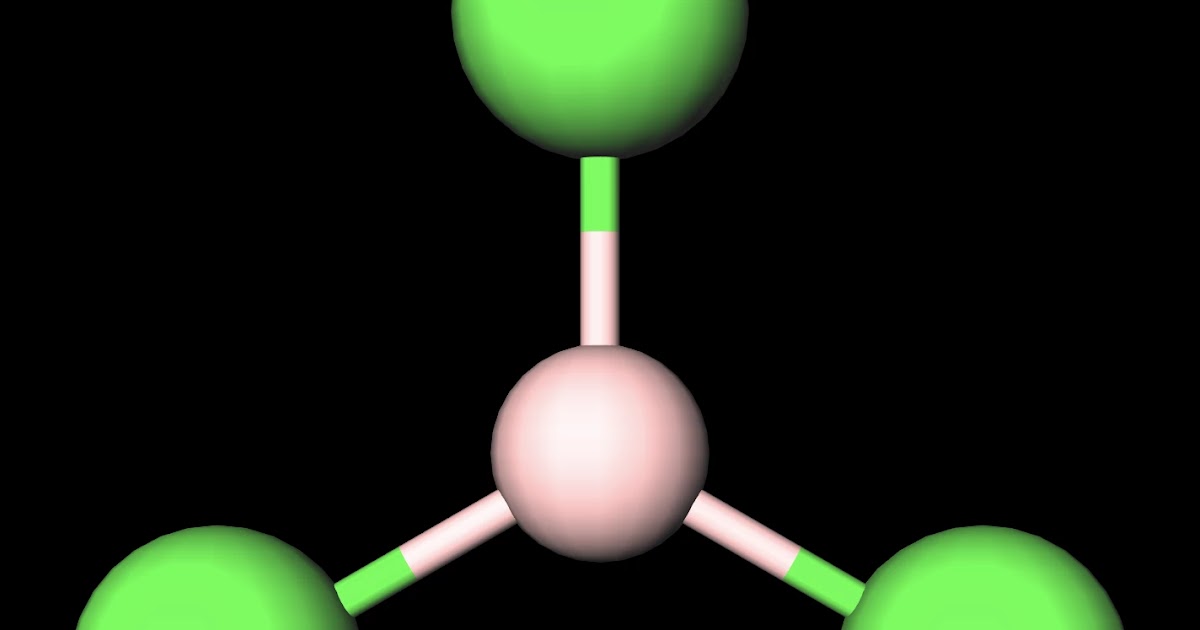
MakeTheBrainHappy Is BCl3 Polar or Nonpolar?
Iodine trichloride (ICl3) is a polar molecule. The central iodine (I) atom in the ICl3 molecule is surrounded by three chlorine (Cl) atoms via single covalent bonds, forming an asymmetric T-shaped molecule. The electronegativity of the chlorine (Cl) atom is greater than the iodine (I) atom.

Opcl3 Lewis Structure
ICl3) molecule is classified as a polar molecule. The molecule of iodine trichloride (with trigonal bipyramidal molecular geometry) is tilted, the bond angles between iodine and. Molecules can be classified as polar or nonpolar. The molecule polar behaves in a different manner as compared to nonpolar. Overview:

POCl3 Lewis Structure (Phosphoryl Chloride) Lewis, Math, Molecules
A. Is ICl3 polar or nonpolar? B. Is PF polar or nonpolar? C. Is SF6 polar or nonpolar? Submit Answer Try Another Version 1 item attempt remaining pl PE
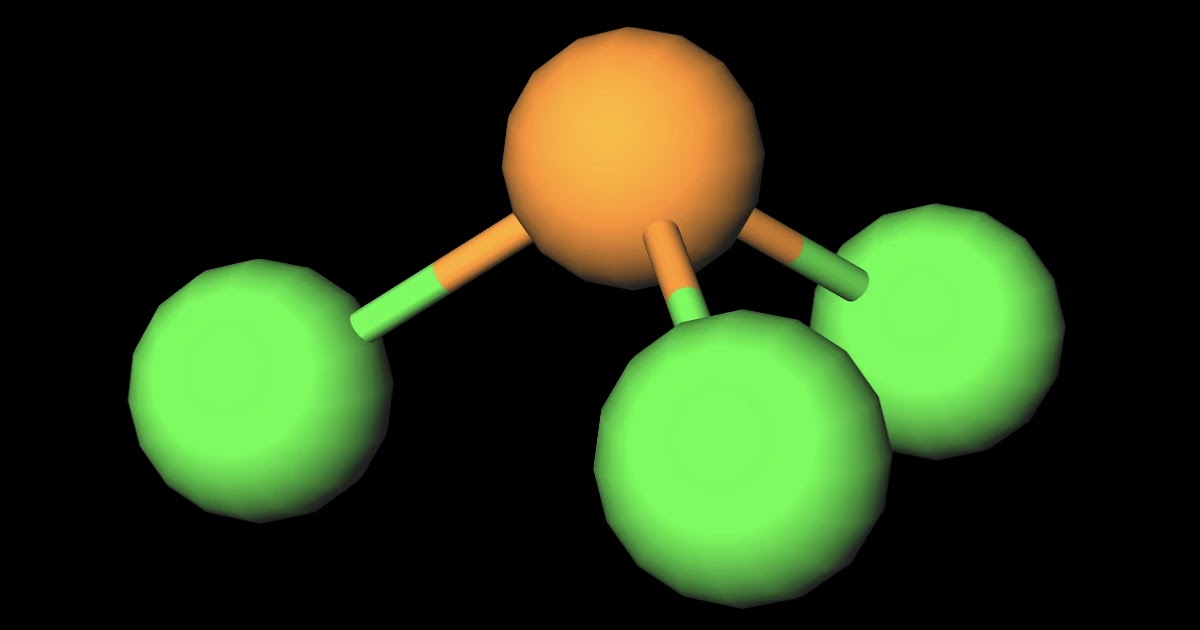
MakeTheBrainHappy Is PCl3 Polar or Nonpolar?
A small difference (<0.4) results in a nonpolar covalent bond, an intermediate difference (0.4 - 1.7) results in a polar covalent bond, and a large difference (>1.7) results in an ionic bond. According to the figure above, a difference in electronegativity (\(\Delta\) EN) greater than 1.7 results in a bond that is mostly ionic in character.

Polar vs. Nonpolar Bonds — Overview & Examples Expii Ionic Bonding
ICl3 has a trigonal bipyramid structure and it is highly symmetrical. The Cl are in the in the x,y plane in a radial symmetry and the both lone pairs are in z plane in opposition of each other. So all of the dipole moments created by the bonds have a net zero direction because of symmetric opposition. 6
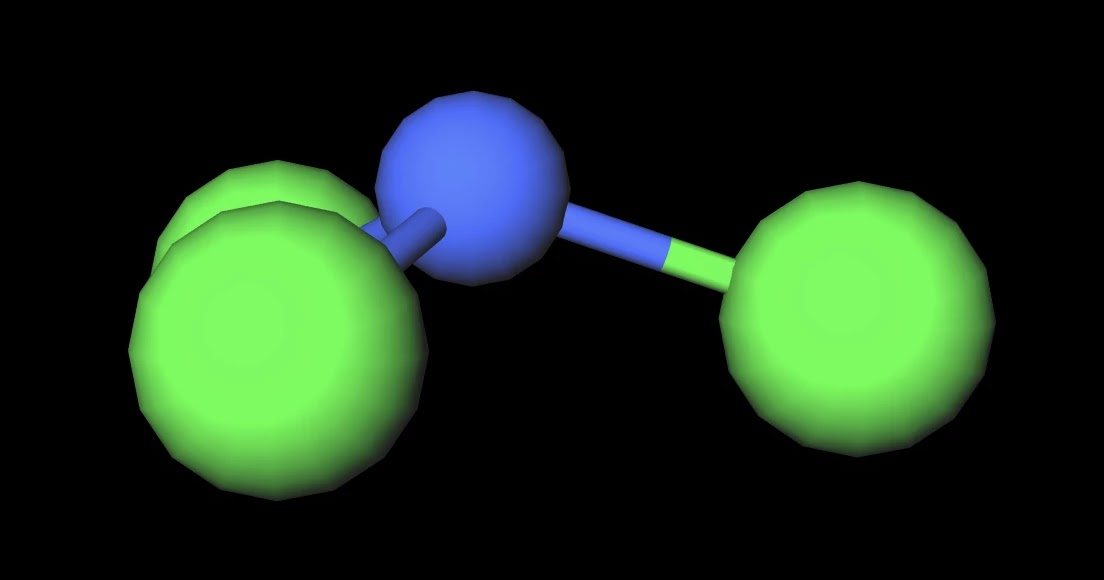
MakeTheBrainHappy Is NCl3 Polar or Nonpolar?
ICl or iodine monochloride is an interhalogen compound which is formed by reaction with chlorine and iodine. It's boiling point and melting point is 97.40C and 27.20C respectively. It is a reddish brown colored substance which is corrosive in nature. Iodine chloride is used as source of electrophilic iodine, preparing iodates.

Best Overview Is CH3F Polar or Nonpolar Science Education and Tutorials
Which of these molecules and ions contain polar bonds? Which of these molecules and ions have dipole moments?ICl3OpenStax™ is a registered trademark, which w.
MakeTheBrainHappy Is SCN Polar or Nonpolar?
Is ICl3 polar or nonpolar ? Question = Is ICl3 polar or nonpolar ? Answer = ICl3 (Iodine trichloride) is Polar What is polar and non-polar? Polar "In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment.