
C2H4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram
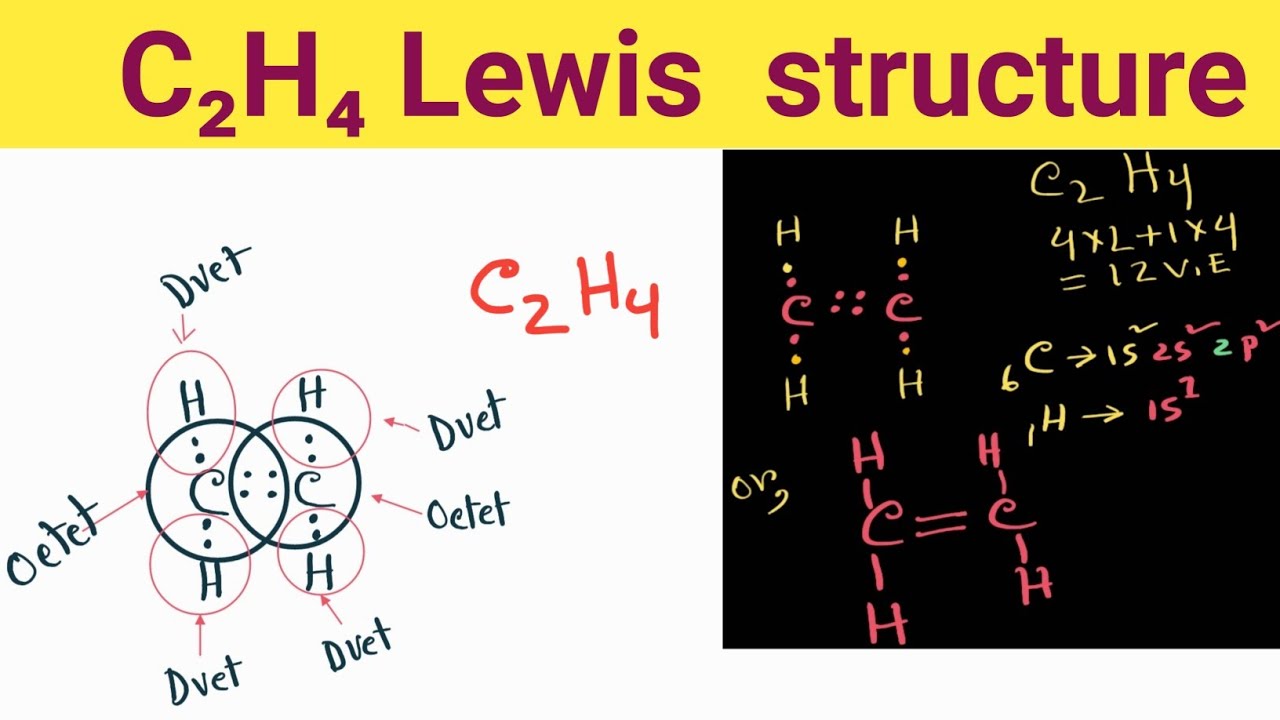
Lewis structure of C2H4. The information on this page is fact-checked. The Lewis structure of C2H4 contains one double bond and four single bonds, with two carbons in the center, and each carbon is attached with two hydrogens. Both hydrogen atom and carbon atom do not have any lone pair.

いします OFFWHITE by たくま's shop|オフホワイトならラクマ C2H4の通販 ジャケット
A quick explanation of the molecular geometry of C2H4 including a description of the C2H4 bond angles.Looking at the C2H4 Lewis structure we can see that the.

C2H4 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram
Chemical Bonding: Lewis Dot Structure for C2H4 (6 of 6) Watch the video of Dr. B. drawing the Lewis dot structure for C2H4 (ethene) and answer the questions below. Note that the C 2 H 4 Lewis dot structure involves sharing more than one pair of electrons. Hint: look at the questions before watching the video.

C2h4 ethylene molecule Royalty Free Vector Image
The Lewis structures and models of methane, ethane, and pentane are illustrated in Figure 20.2. Carbon chains are usually drawn as straight lines in Lewis structures, but one has to remember that Lewis structures are not intended to indicate the geometry of molecules. Notice that the carbon atoms in the structural models (the ball-and-stick and.

draw lewis structures for the ethylene molecule ( c2h4 ), the
C2H4 (Ethene) lewis structure has a double bond between the two Carbon atoms (C) and a single bond between the Carbon atom (C) and Hydrogen atom (H). If you haven't understood anything from the above image of C2H4 lewis structure, then just stick with me and you will get the detailed step by step explanation on drawing a lewis structure of C2H4 .

C2f4 Lewis Structure
C2H4 Lewis structure contains four C-H bonds and one double bond in between two carbon atoms. No lone pair is present on the central or outer atom in the lewis structure of C2H4. The lewis dot structure of C2H4 is very easy to draw- Some steps need to follow for drawing the C2H4 Lewis dot structure 1. Count total valence electron in C2H4
C2H4 Lewis StructureHow do you draw the Lewis structure for C2H4
We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 7.9 Lewis symbols illustrating the number of.
C2h4 Lewis Structure
Lewis structure of C2H4 (or Ethene) contains one double bond between the two Carbon (C) atoms and four single bonds between Carbon (C) & Hydrogen (H) atoms. Let's draw and understand this lewis dot structure step by step. (Note: Take a pen and paper with you and try to draw this lewis structure along with me.

C2H4 Lewis Structure (Ethylene) YouTube
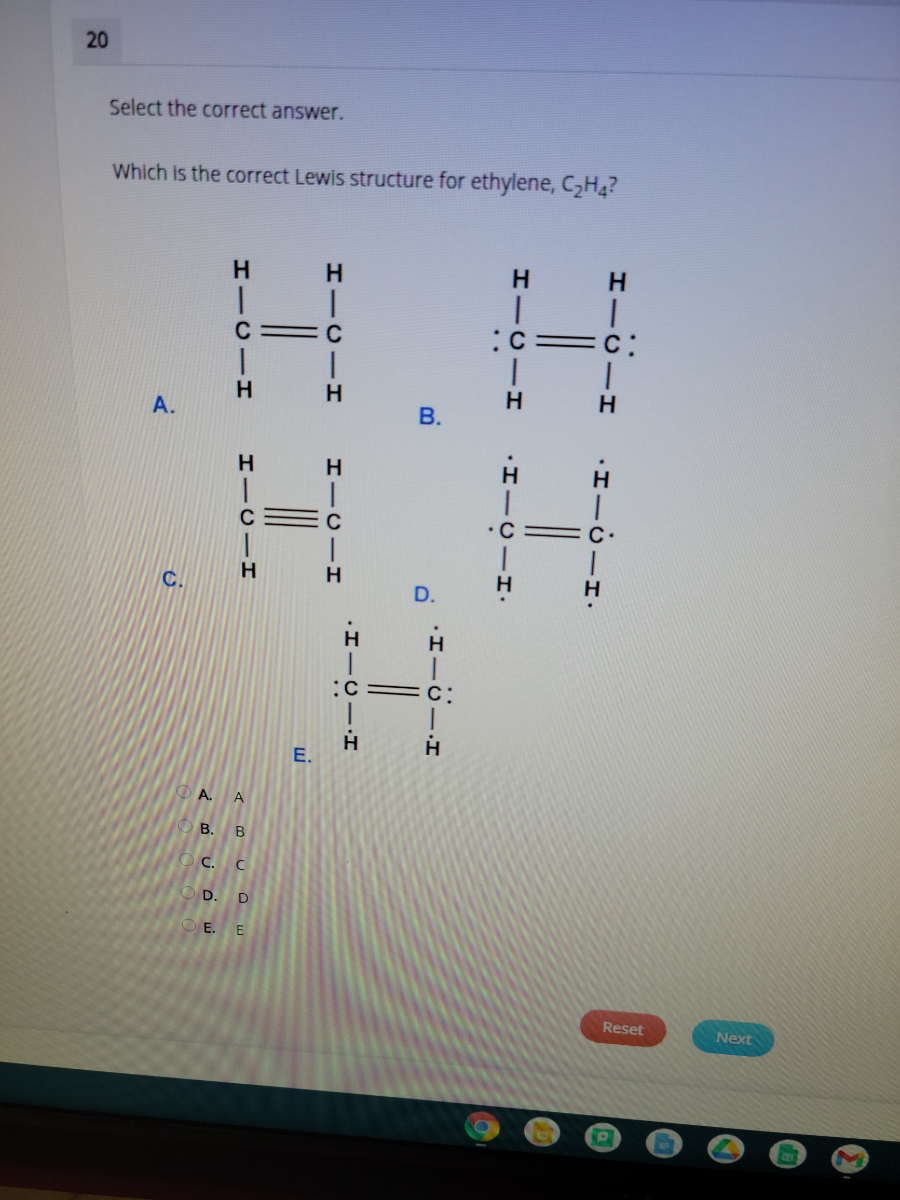
Draw Lewis structures for the following molecular formulas: C2H4 (one double bond), C4H6 (two double bonds), and C4H6 (one triple bond). Mark all non-bonding electrons in each structure.

C2H4 Lewis Structure YouTube
This video teaches you how to draw the Lewis Structures and themoleculargeometry for ethylene (C2H4).

Draw the Lewis structure for C2H4 (whose skeletal structure is H2CCH2
Drawing the Lewis structure for C 2 H 4 (named ethene) requires the use of a double bond. In a double bond two pairs of valence electrons are shared (for a total of four valence electrons). Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence electrons for a full outer shell.

How to Draw the Lewis Dot Structure for C2H4 Ethene YouTube
A step-by-step explanation of how to draw the C2H4 Lewis Dot Structure (Ethene).For the C2H4 structure use the periodic table to find the total number of val.
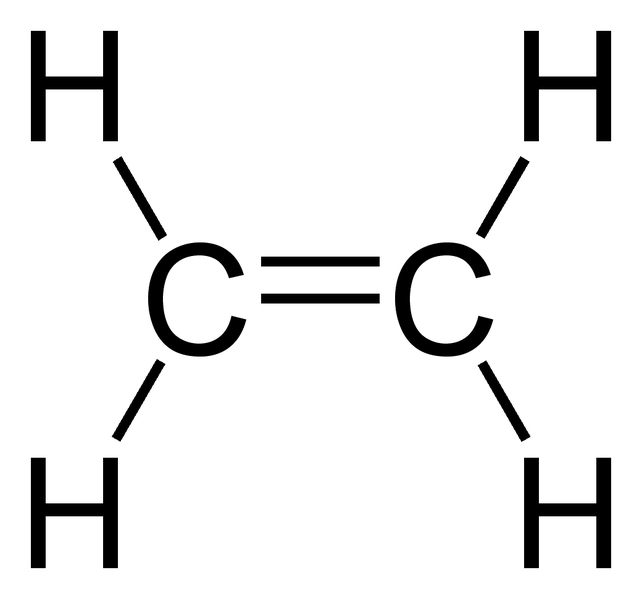
Ethylene C2H4 Dash Model
Contents show Ethylene/Ethene In this article, we will talk about one of the most common and widely used hydrocarbons: Ethylene (C2H4). Do you know that this compound is even lighter than air? Well, C2H4 is a simple straight-chain hydrocarbon that bears a sweet aroma and has a colorless form.

C2H2 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and
C 2 H 4 Lewis structure C 2 H 4 (ethylene or ethene) has two carbon atoms and four hydrogen atoms. In the C 2 H 4 Lewis structure, there is a double bond between the two carbon atoms, and each carbon is attached with one hydrogen atom, and none of the atoms has a lone pair. How to Draw the Lewis Dot Structure for C2H4: Ethene Watch on Contents

C2H4 Molecular Geometry / Shape and Bond Angles YouTube
Hi Guys! Welcome to Geometry of Molecules, and today in this video we are going to help you know the step-by-step method for determining the Lewis Structure.

C2h4 Dot Diagram
Written by Priyanka in Science The chemical formula C2H4 represents Ethylene. This molecule is also represented by H2C=CH2, clearly showing the alkene nature of the compound. An alkene is a hydrocarbon with a Carbon-Carbon double bond. C2H4 exists as a colorless gas and is flammable. Its odor is described as faintly 'sweet and musky'.