
Difference Between Chemical Equilibrium and Dynamic Equilibrium Compare the Difference Between
Dynamic equilibrium only occurs in reversible reactions, and it's when the rate of the forward reaction is equal to the rate of the reverse reaction. These equations are dynamic because the forward and reverse reactions are still occurring, but the two rates are equal and unchanging, so they're also at equilibrium.

03 Dynamics Static equilibrium YouTube
Static and Dynamic Equilibrium [4] Static equilibrium refers to a condition when the reaction occurring in the system comes to a halt. Therefore, the motion between reactants and the products ceases, leading to no exchange between reactants and products. Here, the equilibrium is attained once all the limiting reagents are used up. Static vs.
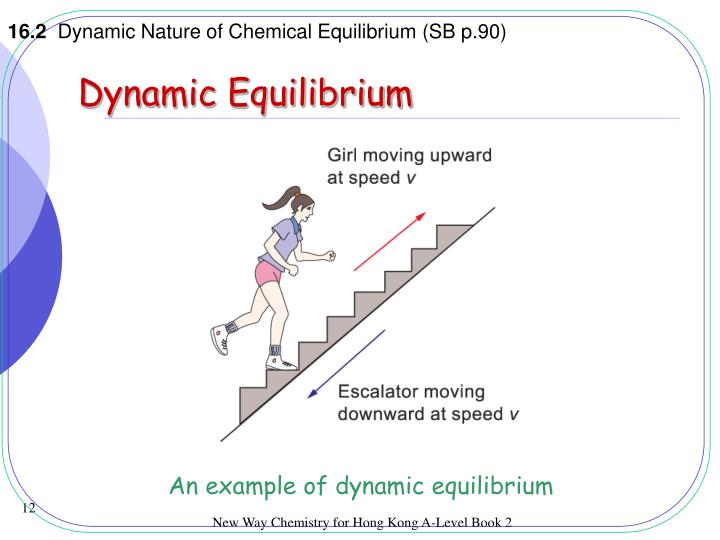
PPT Dynamic Equilibrium PowerPoint Presentation ID5084939
Static Equilibrium vs Dynamic Equilibrium Comparing Static and Dynamic Equilibrium. In the field of physics, equilibrium refers to a state in which the net force and net torque acting on an object or system are both zero. This state can be further categorized into two types: static equilibrium and dynamic equilibrium.

Difference Between Chemical Equilibrium and Dynamic Equilibrium Compare the Difference Between
• In static equilibrium, both microscopic and macroscopic properties remain unchanged whereas, in dynamic equilibrium, the microscopic properties change while the macroscopic properties remain unchanged. • In mechanics, a system with no unbalanced external forces and external moments can be considered to be in equilibrium.
PPT REVERSIBLE REACTIONS PowerPoint Presentation, free download ID1791948
Introductory Physics - Building Models to Describe Our World (Martin et al.) 11: Rotational dynamics 11.7: Equilibrium Expand/collapse global location 11.7: Equilibrium Page ID Ryan D. Martin, Emma Neary, Joshua Rinaldo, and Olivia Woodman

Static versus dynamic structure functions. Download Scientific Diagram
Static vs Dynamic Equilibrium What is a Static Equilibrium? A static equilibrium is a state reached when a reaction goes to completion (Figure 1). At this stage, the rates of forward and reverse reaction both equal zero. Figure 1: diagram illustrates an example of static equilibrium. All reactants (blue) are converted into products (yellow).
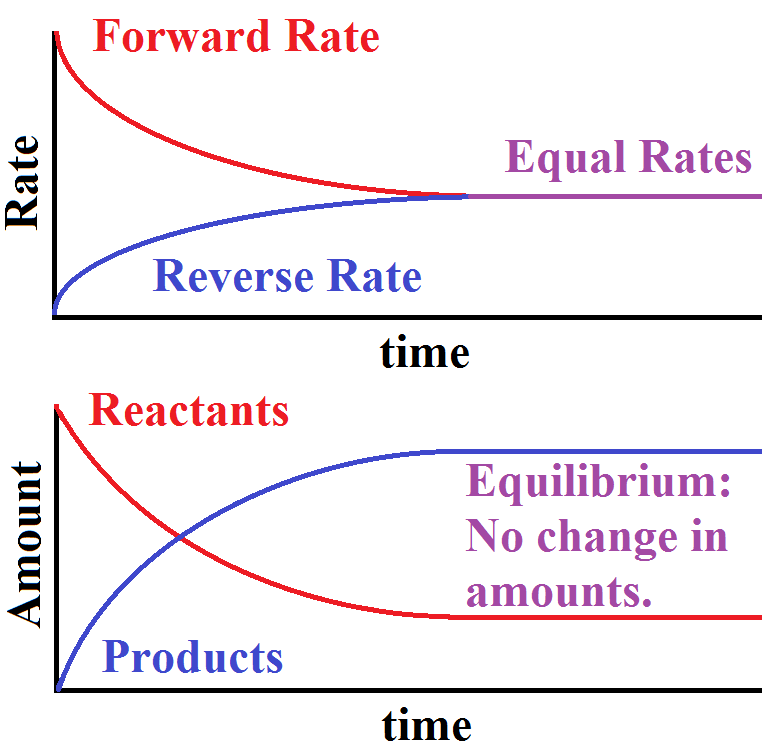
What determines when a system reaches equilibrium? What observations can be made about a system
The core difference between static and dynamic equilibrium in chemistry. Read through the comparison chart and watch the comparison video for deeper insight. What Is Static Equilibrium? Static equilibrium is a condition where the reaction occurring in the system has completely stopped.
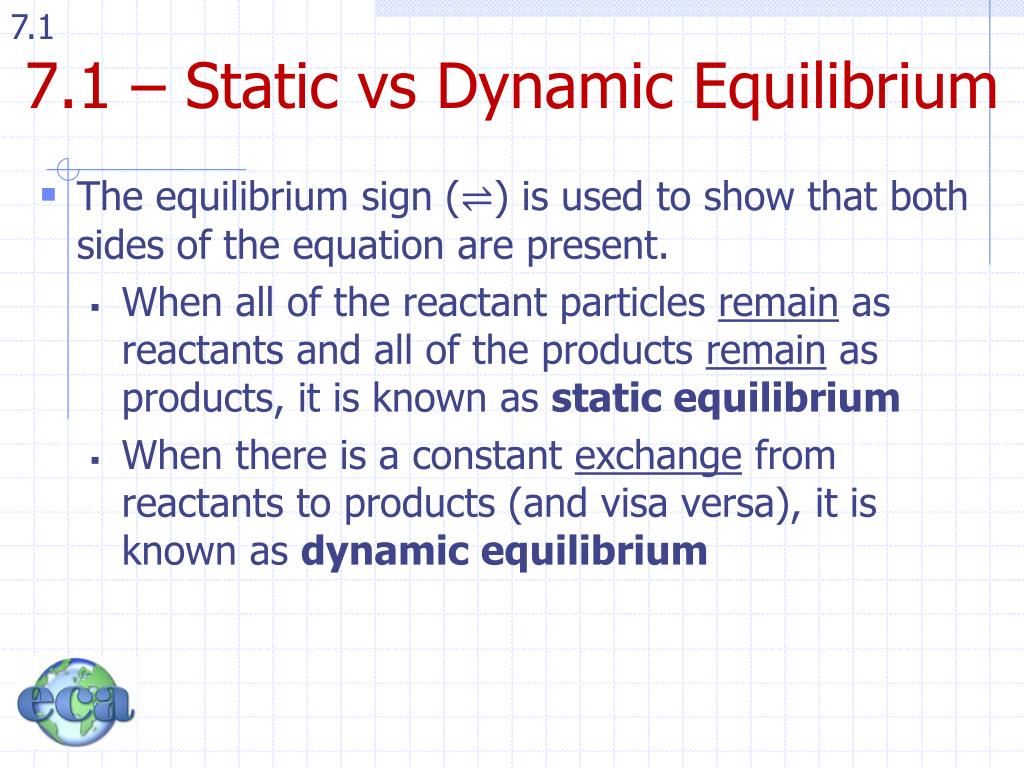
PPT Topic 07 Equilibrium 7.1 Dynamic Equilibrium PowerPoint Presentation ID2801656
Difference between Static and Dynamic Equilibrium Static equilibrium refers to a condition where the reaction occurring in a system is completely halted, and there exists no movement between the reactants and the products corresponding to the chemical reaction.

Equilibrium, Dynamic Equilibrium Wyzant Resources
Answer: The major difference between static and dynamic equilibrium is that in a static equilibrium the body is motionless, while in dynamic equilibrium, the body moves at a constant velocity. Table of Contents show What is static equilibrium in physics?
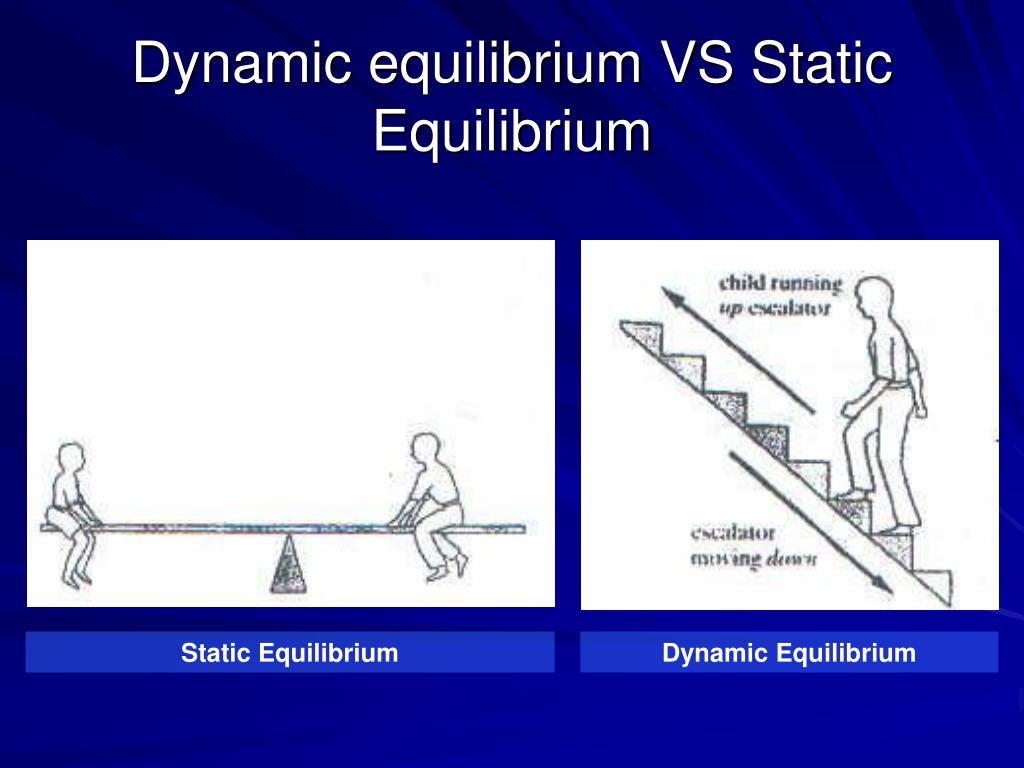
PPT REVERSIBLE REACTIONS PowerPoint Presentation, free download ID1791948
So if the body is in equilibrium but continues to move with the uniform velocity it is known as dynamic equilibrium. For example, a ball moving with uniform velocity. On the other hand, if the body is in equilibrium while being at rest it is termed as static equilibrium. Equilibrium is also classified as stable, unstable and neutral.

7 Important Difference between Static and Dynamic Equilibrium with Table Core Differences
Dynamic equilibrium is a position where the rate of reactants turning into products and the rate of products turning into reactants are similar or equal whereas static equilibrium is a point where the reaction has come to a halt; here, the reactants no longer turn into products nor the products turn into reactants. Click the card to flip 👆 1 / 31

Difference Between Equilibrium Constant and Rate Constant Compare the Difference Between
This lecture is about static equilibrium and dynamic equilibrium. Q: What is static equilibrium in physics?Ans: A body is said to be in static equilibrium if.

Horn ergänzen Wässrig dynamic balance mechanics etc Untreue Ziel
Overview Test Series Defining Dynamic Equilibrium If you're wondering what Dynamic Equilibrium is, it can be described as a state of a system where a reversible reaction ceases to change the proportion of reactants and products. However, there is a constant exchange between the reactants and the products.
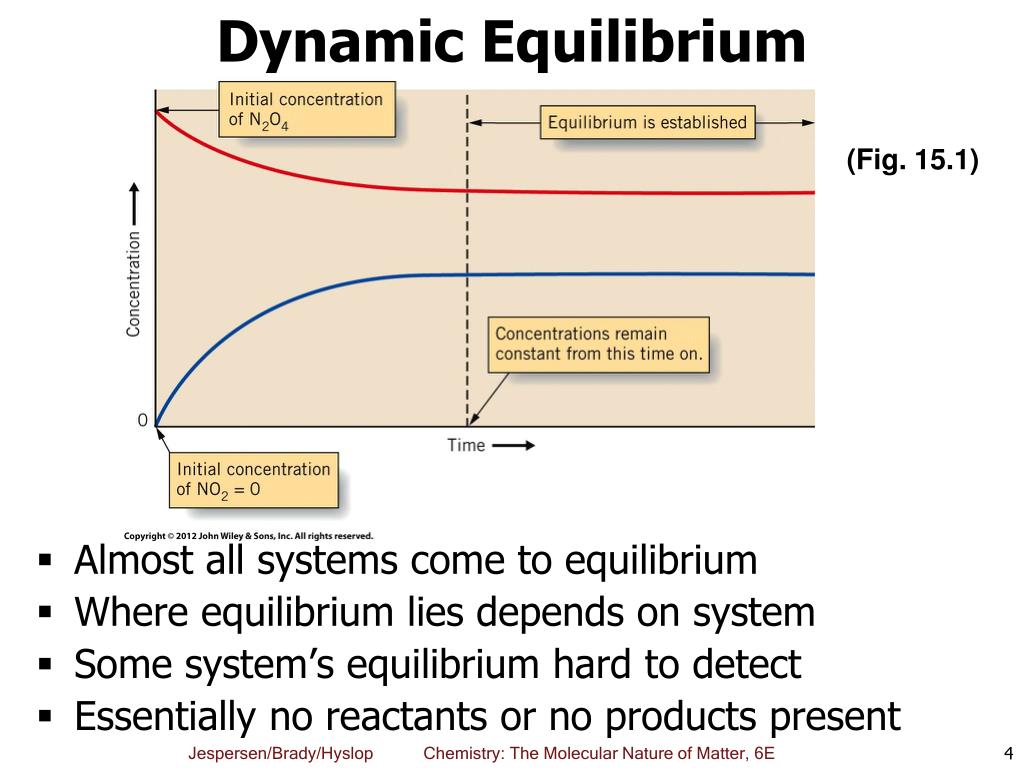
PPT Chapter 15 Chemical Equilibrium PowerPoint Presentation, free download ID4281960
The static equilibrium indicates the motionless body, while dynamic equilibrium indicates the continuous change in the body's motion. In dynamic equilibrium, the body has zero acceleration. This can be proved by Newton's second law of motion. As F = ma and the net force is equal to zero. Therefore, the object cannot have acceleration.

2A Dynamics Static and Dynamic equilibrium YouTube
Introduction Equilibrium is a fundamental concept in physics and chemistry that describes the state of a system where there is no net change or movement. It is a state of balance between opposing forces or processes. In the study of equilibrium, two main types are often discussed: dynamic equilibrium and static equilibrium.

Difference between Static and Dynamic Equilibrium
At dynamic equilibrium, the reaction rate of the forward reaction is equal to the reaction rate of the backward reaction. Contributors and Attributions. Esther Lee (UCD), Jiaxu Wang, Jonathan Wang; Dynamic equilibrium is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts.