/lewis-fc84e3f1452e4aacb2fe023cfff2fa08.jpg)
Diatomic Oxygen Lewis Structure
Center atom selection Mark lone pairs on atoms Mark charges on atoms if there are charges. Check the stability and minimize charges on atoms by converting lone pairs to bonds to obtain best lewis structure. Total number of electrons of the valance shells of H 3 O + There are two elements in hydronium ion; hydrogen and oxygen.

19. Lewis Dot Structure of H3O+ How to Draw Lewis Structures Class
A step-by-step explanation of how to draw the H3O+ Lewis Dot Structure (Hydronium ion).For the H3O+ structure use the periodic table to find the total number.

A stepbystep explanation of how to draw the H3O+ Lewis Structure
For the H3O+ Lewis structure we first count the valence electrons for the H3O+ molecule using the periodic table. Once we know how many valence electrons there are in H3O+ we can distribute them around the central atom and attempt to fill the outer shells of each atom.

H3O+ Lewis Structure Lewis Dot Structure for H3O+ Hydronium ion
A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 7.9 Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table.

Draw a Lewis structure for H3O+ . Include all hydrogen atoms and show
Lewis Structures. Page ID. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms. The goal is to obtain the "best" electron.

H3O+ Lewis Structure, Geometry, Hybridization, and MO Diagram
We can illustrate the formation of a water molecule from two hydrogen atoms and an oxygen atom using Lewis dot symbols: The structure on the right is the Lewis electron structure, or Lewis structure, for H 2 O. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Moreover, by sharing a bonding pair with oxygen.
Lewis Dot Structure for H3O^+ Science, Chemistry ShowMe
Step 1 Basic concepts:- Lewis structure :- The lewis structure is a representation of velance electron in a m. View the full answer Step 2 Unlock Step 3 Unlock Answer Unlock Previous question Next question Transcribed image text: Draw the Lewis structure for the (H3O+) ion. Not the question you're looking for?
H3o Lewis Dot Structure
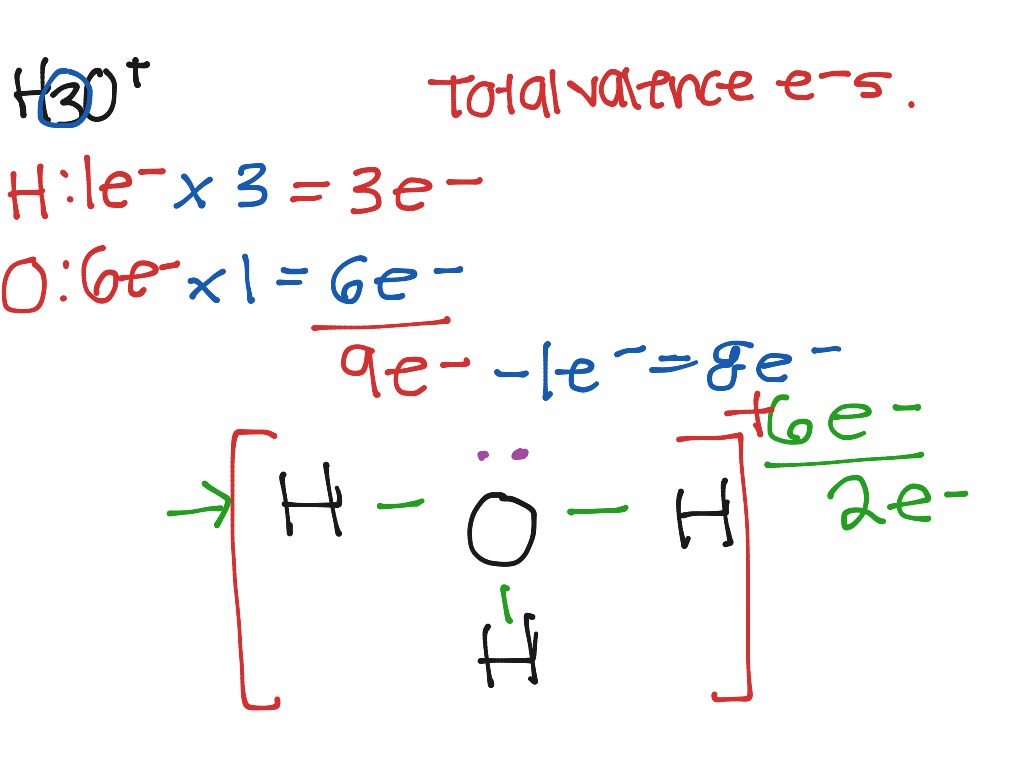
Hydrogen = 1 3*Hydrogen = 3 Oxygen = 6 Total = 9 Now the important point is, not to forget about the + sign. + sign indicates losing an electron from the total valence electrons. ( - sign indicates gaining an electron) Thus, the total valence electron is 8 now.

The Lewis Dot Structure Is Also Called The Electron Dot Diagram
Figure 15.4.3 15.4. 3: The ammonium ion. When drawing the Lewis structure of a polyatomic ion, the charge of the ion is reflected in the number of total valence electrons in the structure. In the case of the ammonium ion: 1 N 1 N atom = 5 = 5 valence electrons. 4H 4 H atoms = 4 × 1 = 4 = 4 × 1 = 4 valence electrons.

How to Draw The Lewis Structure of The Hydronium Ion (H3O+) YouTube
A video explanation of how to draw the Lewis Dot Structure for the Hydronium Ion, along with information about the compound including Formal Charges, Polarit.

H3O+ Lewis Structure (Hydronium Ion) YouTube
1. Count the total valence electrons in [H3O]+ The Lewis dot structure of a molecule is referred to as a simplified representation of all the valence electrons present in it. Therefore, the very first step while drawing the Lewis structure of [H 3 O] + is to find the total valence electrons present in the concerned elemental atoms.
Lewis Dot Structure For H3o Science Chemistry Showme
Lewis Dot Structure of H3O+, (Hydronium Ion) kentchemistry.com 25.1K subscribers Subscribe Subscribed 415 160K views 12 years ago Every Video I quickly take you through how to draw the.

Lewis Dot Structure For H3o Science Chemistry Showme
Let's do the Lewis structure for H3O+, the hydronium ion. On the periodic table, Hydrogen's in group 1, 1 valence electron; but we have 3 Hydrogens. Plus Oxygen, group 6 or 16; 6 valence electrons. And this plus sign means we've lost a valence electron, we've lost a negative charge. So we actually need to subtract 1.

Lewis Structure of Hydronium H3O+ YouTube
Lewis structure of H3O+ ion (Hydronium ion) contains three single bonds between the Oxygen (O) atom and each Hydrogen (H) atom. The Oxygen atom (O) is at the center and it is surrounded by 3 Hydrogen atoms (H). The Oxygen atom has one lone pair and it also has +1 formal charge. Let's draw and understand this lewis dot structure step by step.
Media Portfolio
Step #1: Draw the lewis structure Here is a skeleton of H3O+ ion lewis structure and it contains three O-H bonds. (Note: If you want to know the steps of drawing the H3O+ lewis dot structure, then visit this article: H3O+ lewis structure, Or you can also watch this short 2 minute video).
SOLVED Draw an electrondot structure for the hydronium ion, H3O , and
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".