Gallium (Ga) Periodic Table (Element Information & More)
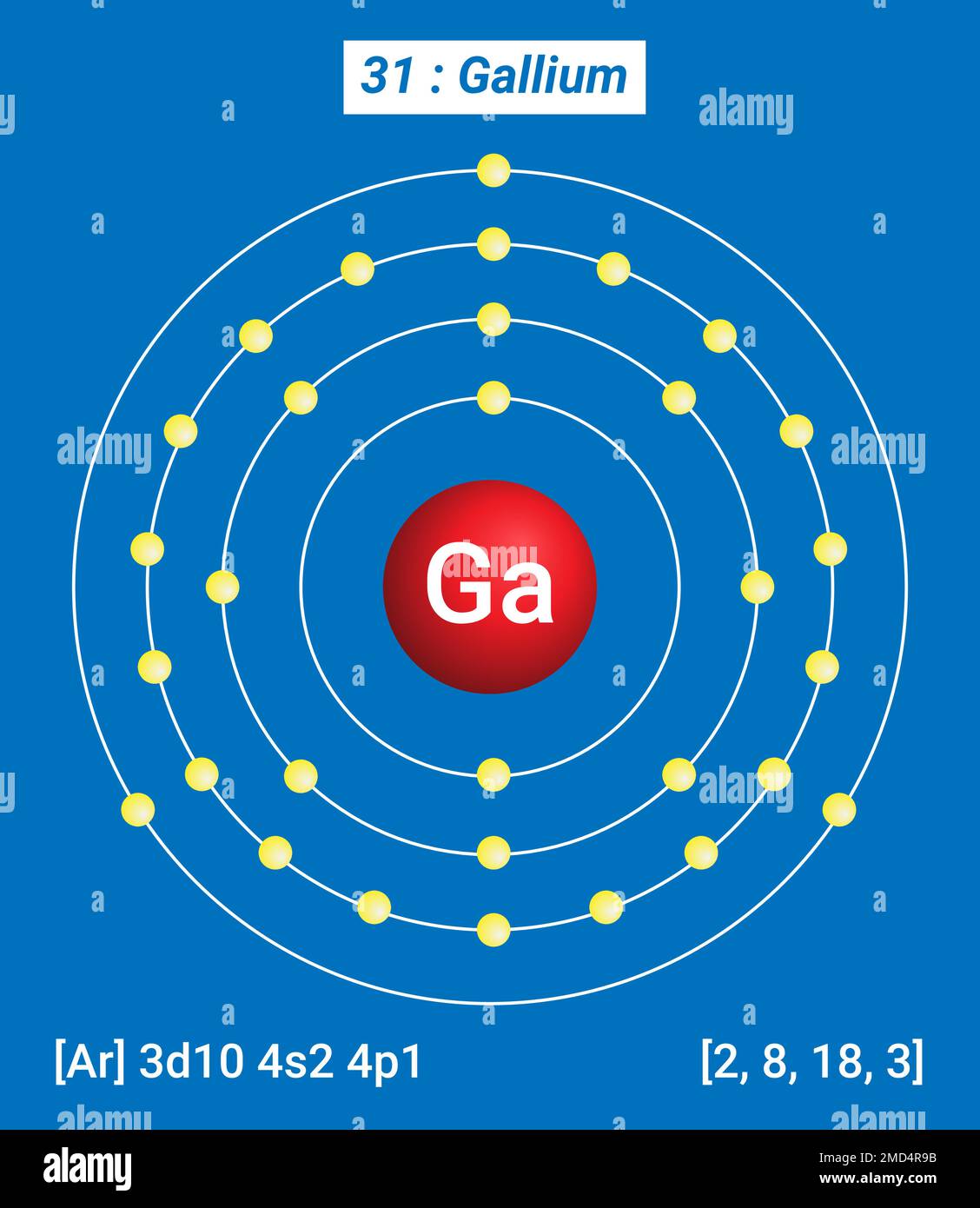
Full electron configuration of gallium: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p1 zinc ← gallium → germanium Gallium, complete electron configuration.

A stepbystep description of how to write the electron configuration
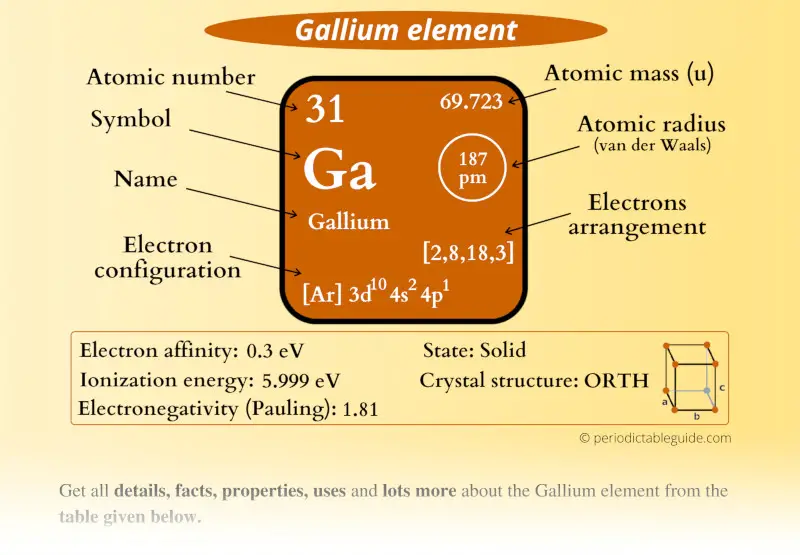
Gallium is the chemical element with the atomic number 31 and symbol Ga on the periodic table. It is in the Boron family (group 13) and in period 4. Gallium was discovered in 1875 by Paul Emile Lecoq de Boisbaudran. Boisbaudran named his newly discovered element after himself, deriving from the Latin word, "Gallia," which means "Gaul.".
Infographic of the Element of Gallium Stock Vector Illustration of
267 38K views 3 years ago A step-by-step description of how to write the electron configuration for Gallium (Ga). In order to write the Ga electron configuration we first need to know.
WebElements Periodic Table » Gallium » properties of free atoms
Referring to either Figure 2.6.3 2.6. 3 or 2.6.4 2.6. 4, we would expect to find the electron in the 1 s orbital. By convention, the ms = +1 2 m s = + 1 2 value is usually filled first. The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2.

Symbol and electron diagram for Gallium Royalty Free Vector
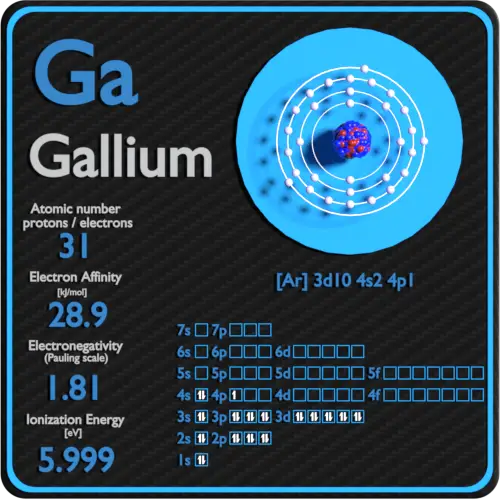
In the above electron configuration, the highest energy level (4) is marked with green color. The 4 th energy level contains 4s and 4p subshells. There are 2 electrons in the 4s subshell and 1 electron in the 4p subshell. So gallium has a total of 2 + 1 = 3 valence electrons. Your feedback matters.
Gallium Ga (Element 31) of Periodic Table Element FlashCards
Gallium is a chemical element; it has symbol Ga and atomic number 31.. [Xe]4f 14 5d 10 6s 2 electron configuration, which is liquid at room temperature. The 3d 10 electrons do not shield the outer electrons very well from the nucleus and hence the first ionisation energy of gallium is greater than that of aluminium.
Ga Gallium, Periodic Table of the Elements, Shell Structure of Gallium
Here, the electron configuration of gallium ion(Ga 3+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10. This gallium ion(Ga 3+) has thirty-one protons, thirty-nine neutrons, and twenty-eight electrons. Gallium ion: Protons: Neutrons: Electrons: Ga 3+ 31: 39: 28: Number of protons, neutrons and electrons for the gallium ion(Ga 3+)

Gallium Electron configuration Symbol Atomic Number Atomic Mass
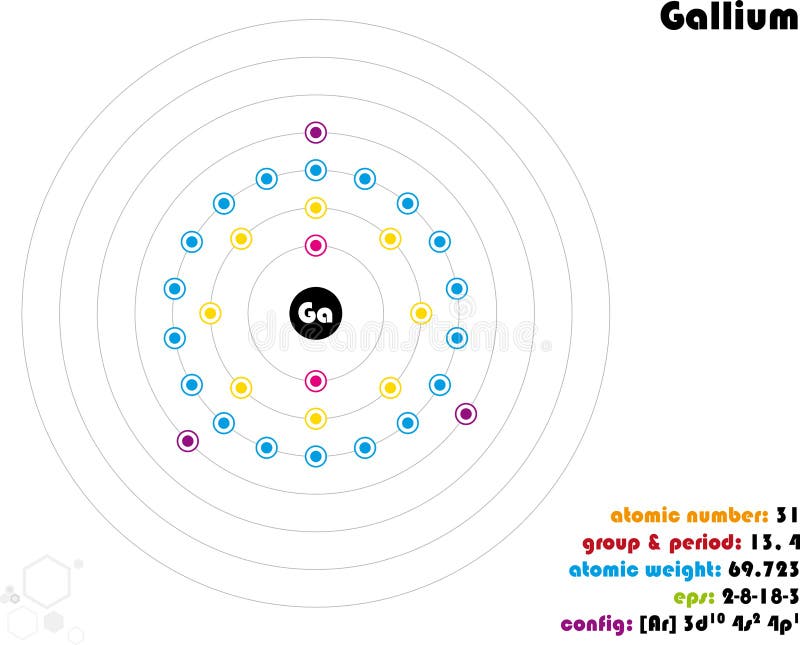
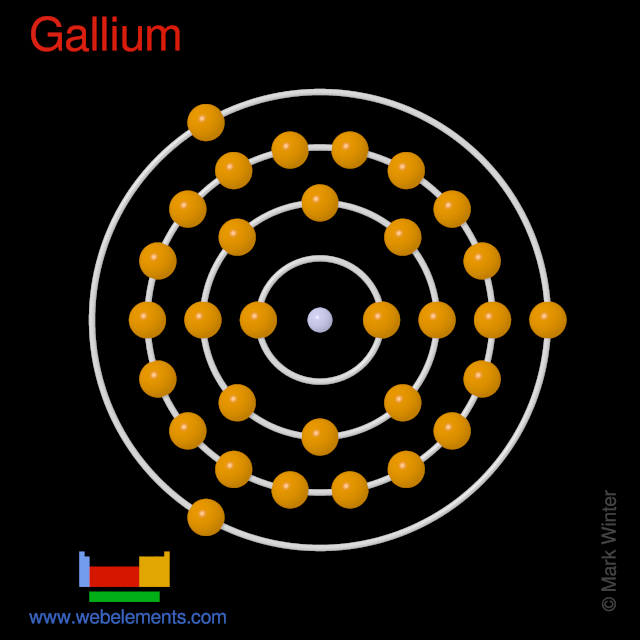
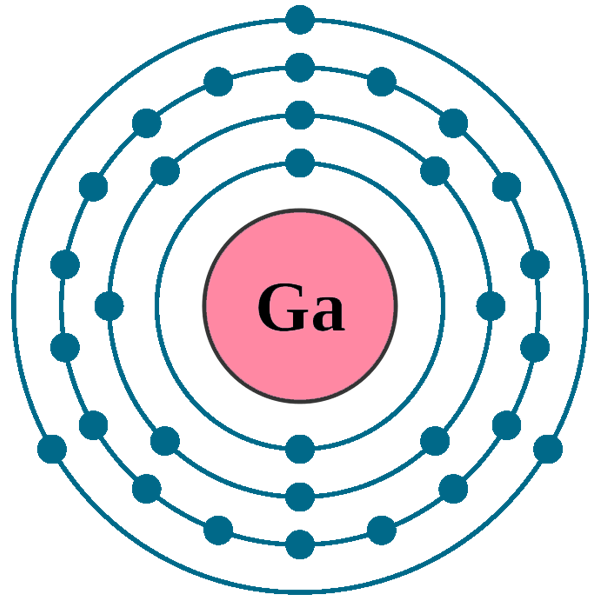
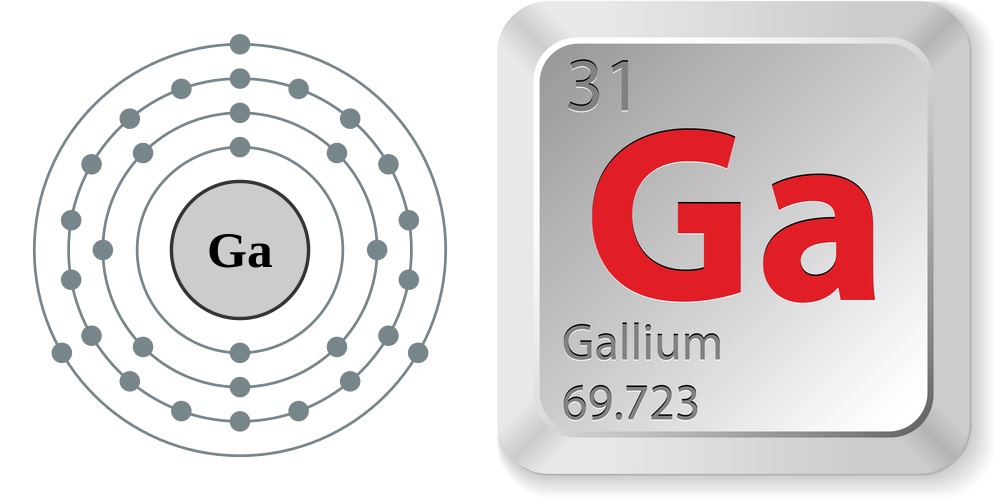
We know that gallium atoms have a total of thirty-one electrons. The electron configuration shows that there are two electrons in the K shell, eight in the L shell, eighteen in the M shell, and three in the N shell.

Electron Configuration for Gallium (Ga, Ga3+ ion)
Orbital diagram Ga (Gallium) is an element with position number in the periodic table. Located in the : 29.8 ℃. Electronic configuration of the Gallium atom. Valence electrons. Orbital diagram

Ga electronic configurationHow to write electronic configuration of
The arrangement of electrons in gallium in specific rules in different orbits and orbitals is called the electron configuration of gallium. The electron configuration of gallium is [ Ar] 3d 10 4s 2 4p 1 , if the electron arrangement is through orbitals. Electron configuration can be done in two ways.

Symbol and electron diagram for gallium Royalty Free Vector
The electron configuration for Gallium, Ga is 1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^1 Gallium, Ga has 31 protons and 31 electrons. The superscripts represent the electrons present in each region of the periodic table. The sum of these superscripts should equal the atomic number for a neutral atom.
Facts About Gallium Live Science
1st shell can hold 2 electrons. 2nd shell can hold 8 electrons. 3rd shell can hold 18 electrons. 4th shell can hold 32 electrons. Now the atomic number of gallium (Ga) is 31. Hence the gallium element has electrons arrangement 2, 8, 18, 3. This electron arrangement indicates that the outermost orbit of Gallium element (Ga) has 3 electrons.

Electron Configuration of Gallium Ga Lesson YouTube
Element Gallium (Ga), Group 13, Atomic Number 31, p-block, Mass 69.723. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images.. Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point
Gallium Periodic Table and Atomic Properties
Gallium-67 (half-life 3.3 days) is a gamma-emitting isotope (the gamma emitted immediately after electron-capture) used in standard nuclear medical imaging, in procedures usually referred to as gallium scans. Stable Isotopes Typical Unstable Isotopes Electrons and Electron Configuration

Gallium (Ga). Diagram of the nuclear composition, electron
#1 Using aufbau principle #2 Using periodic table #3 From its Bohr model #4 From its orbital diagram Let's break down each method in detail. Using aufbau principle First, find electrons of gallium atom Periodic table The atomic number of gallium represents the total number of electrons of gallium.

FileElectron shell 031 Gallium.svg Wikimedia Commons Shells
Step 1: Writing the electron shell number Gallium has 4 electron shells which are written as 1, 2, 3, 4 Step 2: Putting orbital notations after the number of electron shells Gallium atom is consists of 3 orbitals such as 's', 'p' and 'd' Step 3: Calculating the number of electron in orbitals