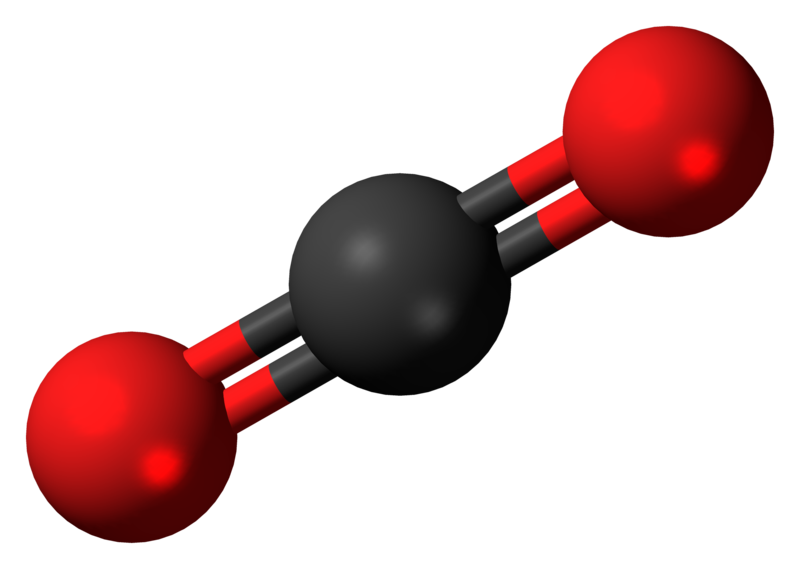
Carbon dioxide (CO2) Climate Encyclopedia
665 109K views 3 years ago This chemistry video explains how to draw the lewis structure of CO2 also known as Carbon Dioxide. It also discusses the bond angle, molecular geometry, and.

CO2 Lewis Structure, Hybridization, Molecular Geometry, and MO Diagram
For Lewis structure of CO2, you will now have two Oxygen atoms forming double bonds with a Carbon atom. As all the valence electrons of all the atoms are used, there are no lone pairs of electrons or non-bonding pairs of electrons in the molecule.

CO2 Molecular Geometry Science Education and Tutorials
There are three resonance structures CO2 (Carbon dioxide). We start with a valid Lewis structure and then follow these general rules. For the CO2 resonance s.

CO2 Lewis Structure ,Valence Electrons, Formal Charge ,Polar or
To draw the Lewis structure of CO2, we first need to determine the number of valence electrons in each atom. Carbon has 4 valence electrons, while each oxygen atom has 6 valence electrons. In total, CO2 has 16 valence electrons. Next, we need to arrange the atoms in the molecule.

CO2 Lewis Structure ,Valence Electrons, Formal Charge ,Polar or
A step-by-step explanation of how to draw the Lewis Dot Structure for Carbon dioxide (CO2 ).For the Carbon dioxide structure we use the periodic table to fi.

What is the Lewis Dot structure for CO2 (Carbon dioxide)?
Lewis Structure of CO2 CO 2 has a total of 16 valence electrons (four for carbon and two for oxygen), which are organized as O=C=O. In their outermost shells, both oxygen and carbon atoms require 8 electrons to complete an octet.

CO2 Lewis Structure ,Valence Electrons, Formal Charge ,Polar or
The Lewis structure helps us identify the bond pairs and the lone pairs. Then, with the Lewis structure, we apply the valence-shell electron-pair repulsion (VSPER) theory to determine the molecular geometry and the electron-group geometry.. Carbon dioxide has two electron groups and no lone pairs. Carbon dioxide is therefore linear in.

The WikiPremed MCAT Course Image Archive A lewis structure showing
Carbon dioxide (CO 2) lewis structure has two oxygen atoms and one carbon atom. There are two double bonds around carbon atom in the CO 2. No lone pairs on carbon atom and each oxygen atom has two lone pairs on their valence shells. Shape of CO 2 is linear. Steps of drawing the lewis structure of CO 2 are explained in detail in this tutorial.
[Solved] 8. Draw the Lewis structures for CO2 and CO, and predict the
The CO2 Lewis structure depicts the molecular arrangement of carbon dioxide, which is composed of one carbon atom and two oxygen atoms. Within the CO 2 Lewis structure, the carbon atom is surrounded by two double bonds, with each oxygen atom attached to it. Additionally, there are two lone pairs on each oxygen atom.

CO2 Lewis Structure ,Valence Electrons, Formal Charge ,Polar or
A step-by-step explanation of how to draw the CO2 Lewis Dot Structure (Carbon dioxide).For the CO2 structure use the periodic table to find the total number.
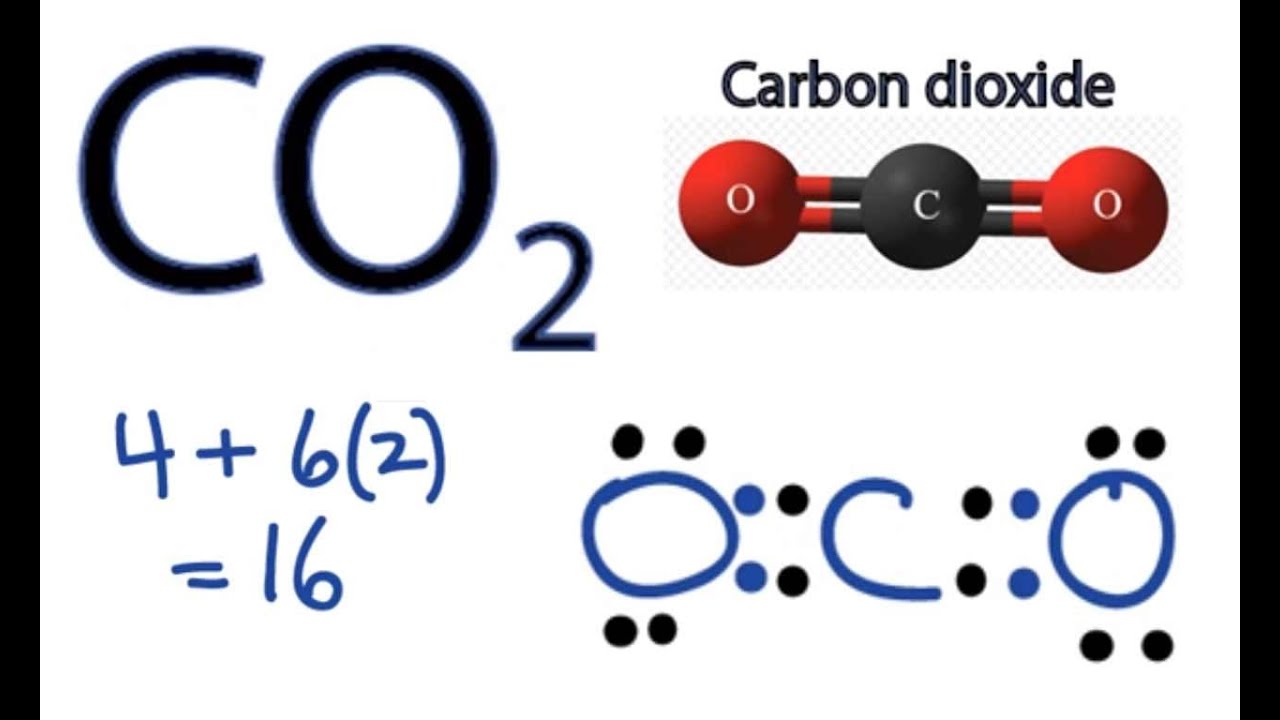
CO2 Lewis Structure How to Draw the Dot Structure for Carbon Dioxide
1: Basic Concepts in Chemical Bonding and Organic Molecules

Molecular Structure of CO2 (Carbon Dioxide) YouTube
Steps of drawing CO2 lewis structure Step 1: Find the total valence electrons in CO2 molecule. In order to find the total valence electrons in CO2 (carbon dioxide) molecule, first of all you should know the valence electrons present in carbon atom as well as oxygen atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.)

What is the Lewis Dot structure for CO2 (Carbon dioxide)?
The CO 2 Lewis structure is symmetric. Generally, small symmetric molecules are nonpolar. CO 2 is a nonpolar substance, meaning it tends to be a gas. CO 2 has a rather low boiling point of around -80 ℃ or -100 ℉. It can be liquified and even frozen solid with special machinery to produce "dry ice.". Dry ice exhibits a temperature around.
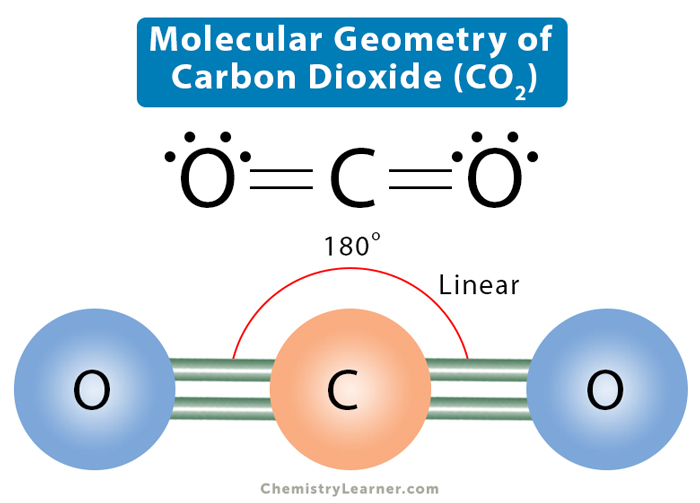
Molecular Geometry, Lewis Structure, and Bond Angle of CO2
The Lewis structure indicates that each Cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written between the atoms). A dash (or line) is sometimes used to indicate a shared pair of electrons: A single shared pair of electrons is called a single bond.
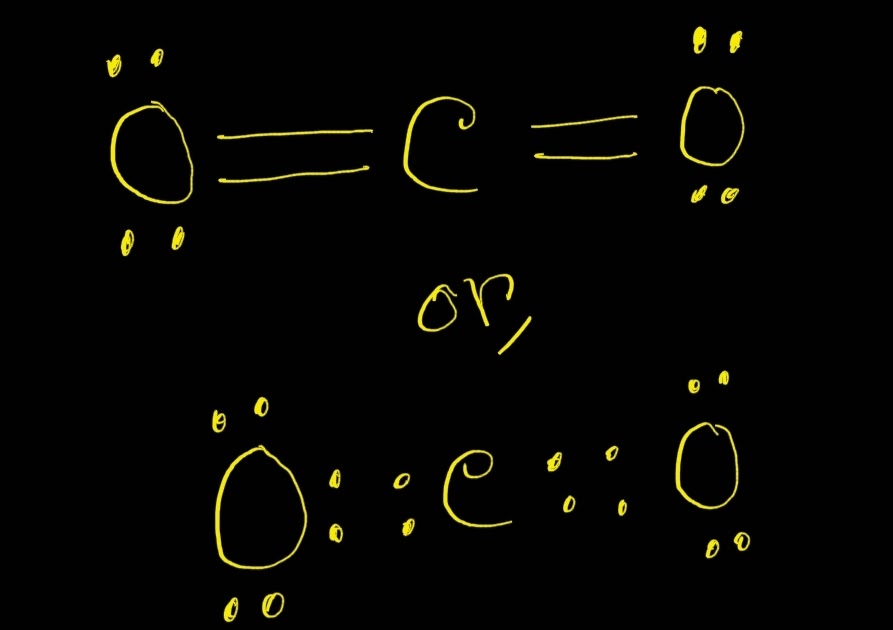
CO2 Lewis Structure ,Valence Electrons, Formal Charge ,Polar or
Lewis Structure Co2 Lewis Structure of Carbon Dioxide Carbon dioxide is a colourless, odourless, incombustible gas produced by the combustion of carbon. The carbon-oxygen ratio in a CO 2 molecule is 1:2. Two double bonds connect the carbon and oxygen atoms in the Lewis structure.

So far, we’ve used 16 of the CO2 Lewis structure’s total 16 outermost
By Biswarup Chandra Dey This article contains detailed facts about 13 important factors of CO2 including CO2 lewis structure, bond angle, shape, etc. In the CO2 lewis structure, the shape of the molecule is linear. All the atoms of CO2 molecule lie in the same plane.