
C2h5oh Lewis Dot Structure
C2H5OH (Ethanol) lewis structure has Carbon atoms (C) at the center which is surrounded by Hydrogen atoms (H) and one OH group. There are five C-H bonds, one O-H bond and one C-O bond. There are 2 lone pairs on the Oxygen atom (O).

C2h5oh Lewis Dot Structure
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".

C2h5oh Lewis Dot Structure
This structure will help us understand the properties of molecules along with their shape and molecular geometry. So first, we will determine the central atom for this molecule. Generally, the least electronegative atom takes the central position. Here Carbon is the least electronegative atom.
C2h5oh Ethanol Molecule Royalty Free Vector Image Free Nude Porn Photos
Count the total number of electrons in the hypothetical structur e. If it is EQUAL to the number of available electrons from step 1, this is the correct Lewis Dot Structure. If it is GREATER than the number of available electrons from step 1, go to step 5. If it is LESS than the available number of electrons from step 1, go to step 7.

C2h5oh Lewis Dot Structure
A dot structure is any representation of atoms/molecules using dots for electrons. And a Lewis diagram (or Lewis structure or Lewis dot structure) is a type of dot structure created by the chemist Gilbert N. Lewis which is most commonly used in chemistry nowadays. There's a slight difference, but they effectively mean the same thing.

C2h5oh Lewis Dot Structure
Step #1: Draw the lewis structure Here is a skeleton of C2H5OH lewis structure and it contains C-H bonds, C-O bond, C-C bond and O-H bond. (Note: If you want to know the steps of drawing the C2H5OH lewis dot structure, then visit this article: C2H5OH lewis structure, Or you can also watch this short 2 minute video).

C2h5oh Lewis Dot Structure
In order to draw the lewis structure of C2H5OH, first of all you have to find the total number of valence electrons present in the C2H5OH molecule. (Valence electrons are the number of electrons present in the outermost shell of an atom). So, let's calculate this first. Calculation of valence electrons in C2H5OH For Carbon:

C2h5oh Lewis Dot Structure
Lesson 4: Dot structures and molecular geometry. Drawing dot structures. Drawing Lewis diagrams. Worked example: Lewis diagram of formaldehyde (CH₂O) Worked example: Lewis diagram of the cyanide ion (CN⁻) Worked example: Lewis diagram of xenon difluoride (XeF₂) Exceptions to the octet rule. Counting valence electrons.

C2H5OH Lewis Structure, Molecular Geometry, Hybridization, and Polarity
Draw a Lewis structure for ethanol (C 2 H 5 OH), then answer the following questions about the structure: Number of valence electrons: Number of bonding electrons: Number of non-bonding electrons: Tries 4/99 Previous Tries Identify the fault, if any, in each of the following Lewis structures for ethanol.If more than one answer appl A Incorrect: Wrong molecular formula.
C2h5oh Lewis Dot Structure
CH 3 CH 2 OH or C 2 H 5 OH or C 2 H 6 O (ethanol) has two carbon atoms, six hydrogen atoms, and one oxygen atom. In the ethanol Lewis structure, there are five C — H bonds, one C — C bond, one C — O bond, and one O — H bond. And on the oxygen atom, there are two lone pairs. CH3CH2OH Lewis Structure: How to Draw the Lewis Structure for CH3CH2OH
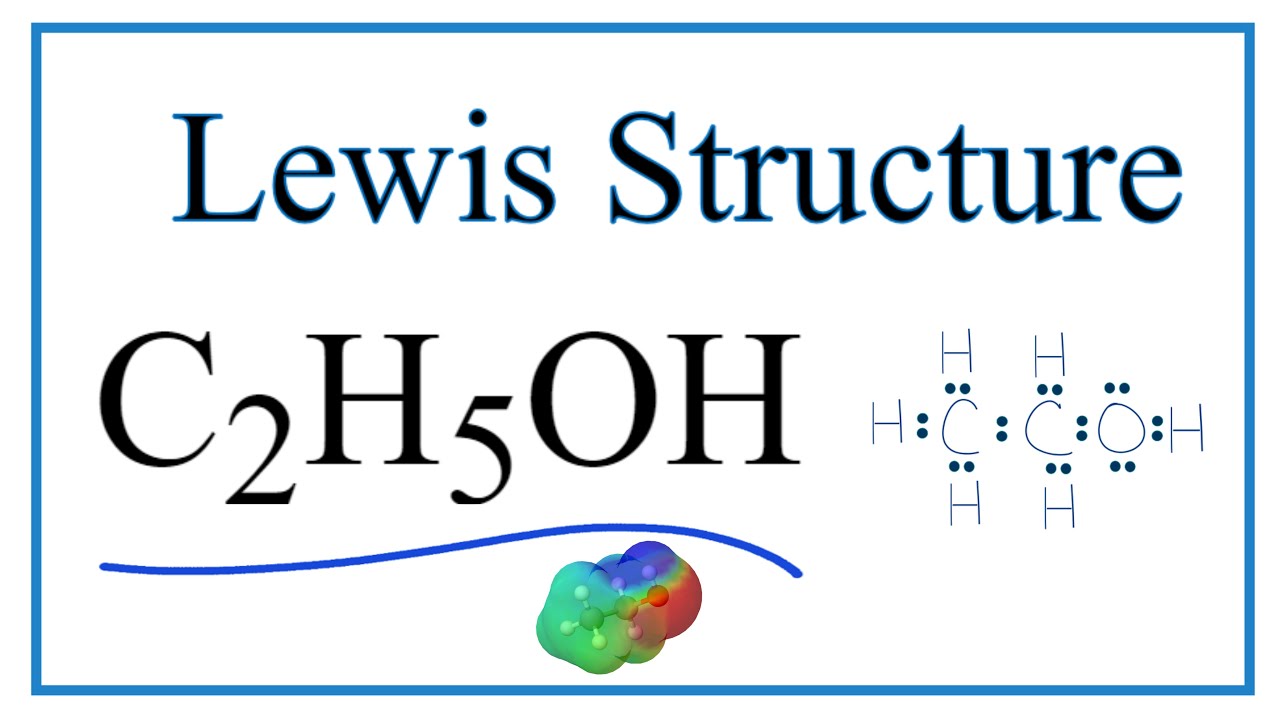
C2h5oh Lewis Dot Structure
Lewis dot structure, also known as electron dot structure or Lewis structure, is a visual representation of the arrangement of atoms and valence electrons in a molecule. It provides valuable insights into the chemical bonding and molecular geometry of a compound.

C2H5OH Lewis Structure (Ethanol) YouTube
Steps for drawing the Lewis dot structure of C2H5OH 1. Count the total valence electrons in C2H5OH The very first step while drawing the Lewis structure of C 2 H 5 OH is to calculate the total valence electrons present in its concerned elemental atoms.
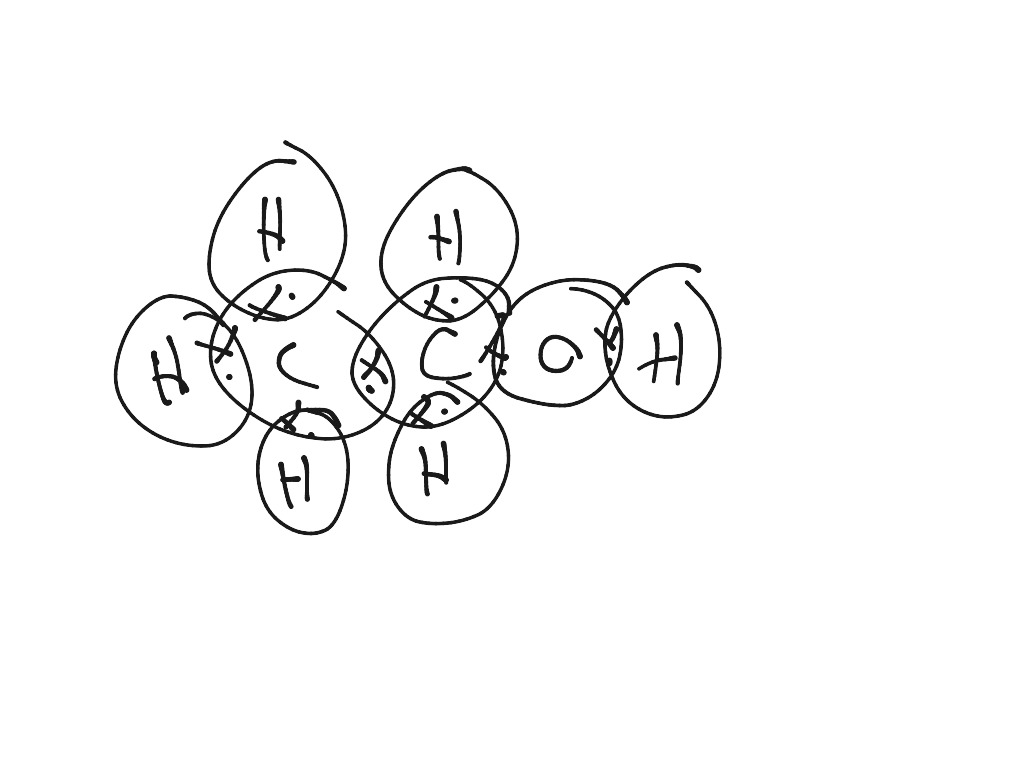
C2h5oh Lewis Dot Structure
A step-by-step explanation of how to draw the C2H5OH Lewis Dot Structure (Ethanol (Ethyl alcohol)).For the C2H5OH structure use the periodic table to find th.

C2h5oh Lewis Dot Structure
Follow these simple steps to draw Lewis dot structures: Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Be sure to have the correct number of electrons. If the species is an ion, add or subtract electrons corresponding to the charge of the ion. Add an electron for every negative (-) charge, and.
Vieme chémiu
This widget gets the Lewis structure of chemical compounds. Send feedback | Visit Wolfram|Alpha Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.

C2h5oh Lewis Dot Structure
Use information from step 4 and 5 to draw the lewis structure. Lewis dot structure of C 2 H 5 OH. Alternatively a dot method can be used to draw the lewis structure. Calculate the total valence electrons in the molecule. C:4x2=8 H:1x6=6 O:6. Total=20 i.e . 10 pairs