
Trigonal Bipyramidal Molecular Geometry Trigonal Planar Molecular Geometry Pentagonal
A trigonal bipyramidal molecule is a bit more complicated, mostly because it has more atoms and bonds to take into account. As the name implies, a trigonal bipyramidal shape looks like two three.

Trigonal Bipyramidal Lewis Structure
1. We know that the structure of CHX4 C H X 4 is tetrahedral, and not planar tetragonal, as a tetrahedron enables maximum distribution of H H -atoms in space, as they repulse from each other. This is proved by the fact that each H−C−H H − C − H bond angle is equal in magnitude. In the case of PClX5 P C l X 5, why should the shape be.

Trigonal Bipyramidal Collection
Molecular Geometry of the Trigonal Bipyramidal Structures. The order of most repulsion to least repulsion among bonding and lone pair electrons are: Lone pair-Lone pair > Lone pair-Bond pair > Bond pair-Bond pair. To decide where to place lone pairs on the parent Trigonal Bipyramidal structure, we must place lone pairs far away from each other.
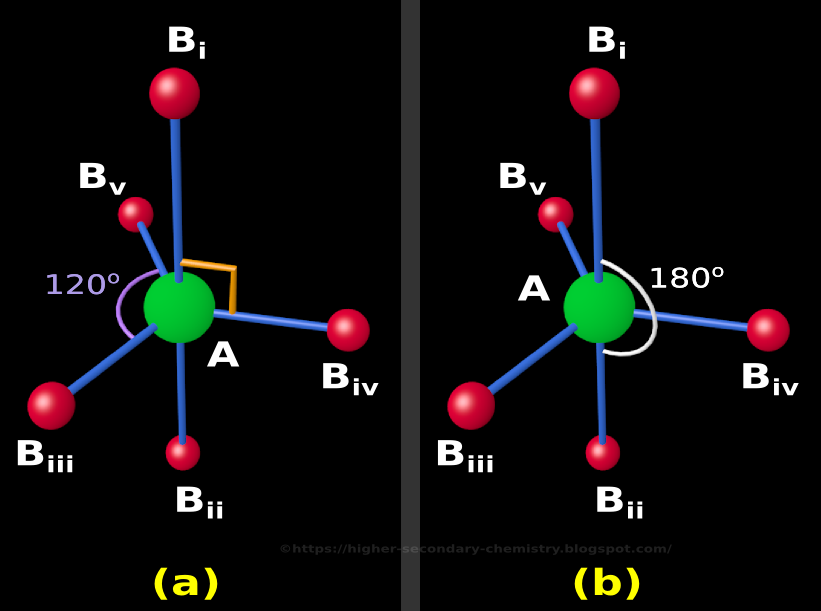
Higher Secondary Chemistry Chapter 4.15 The Trigonal Bipyramidal Structure in VSEPR Theory
Note that the largest angle is ~103 degrees, but the F-P-F angles in the basal plane are all ~87 degrees. Here's the trigonal bipyramidal geometry: Now the angles are exactly what we expect from VSEPR. We see 90 degree angles between the equatorial plane and the axial F atoms. And the F-P-F in the plane are all 120 degrees.
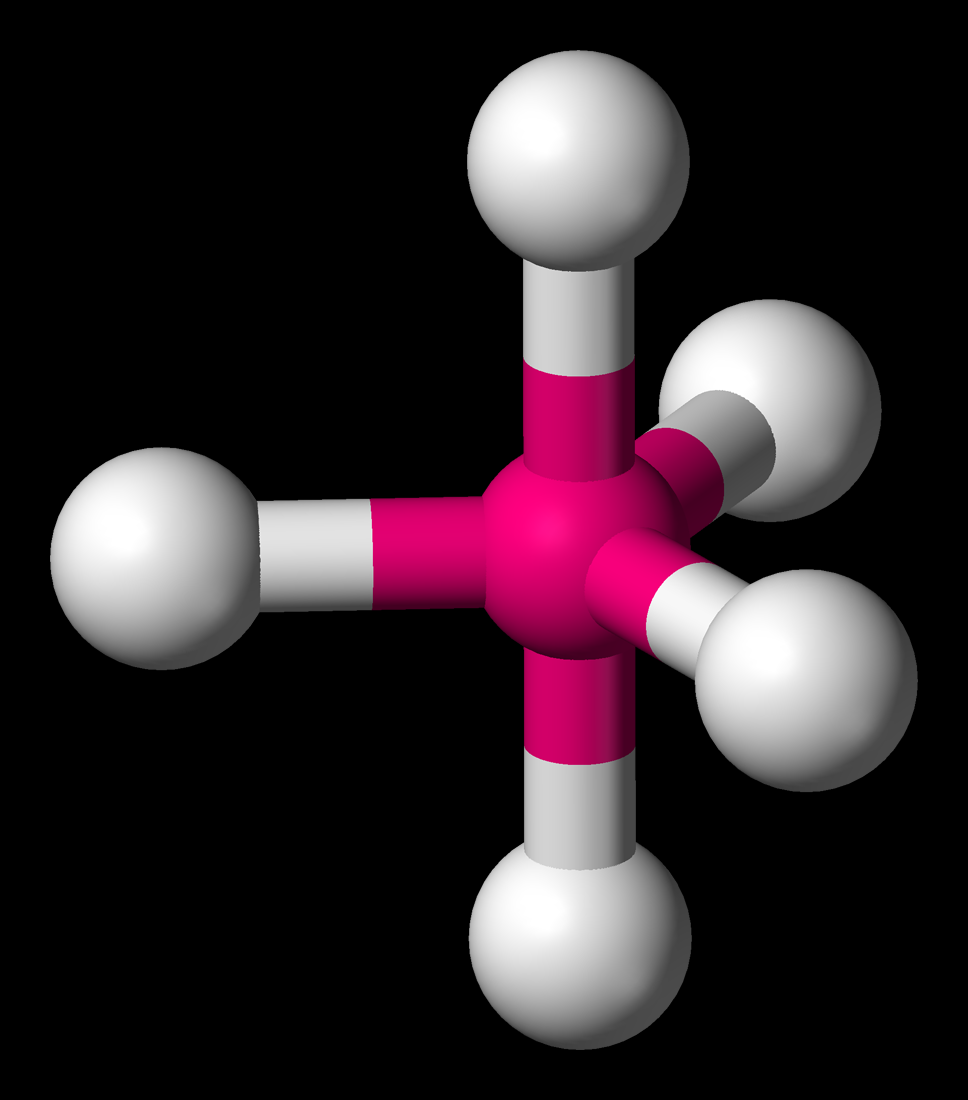
Image Trigonalbipyramidal3D.png Engineering
2.9.1: Trigonal Bipyramidal Species. Page ID. Trigonal Bipyramidal Species are those that have a central p-block atom and are attached to 5 other atoms. It is classified as a EX5 molecule, where E stands for the central atom, and X stands for the atoms that are attached. It makes sense, then, to classify this molecule a 5-coordinate system.
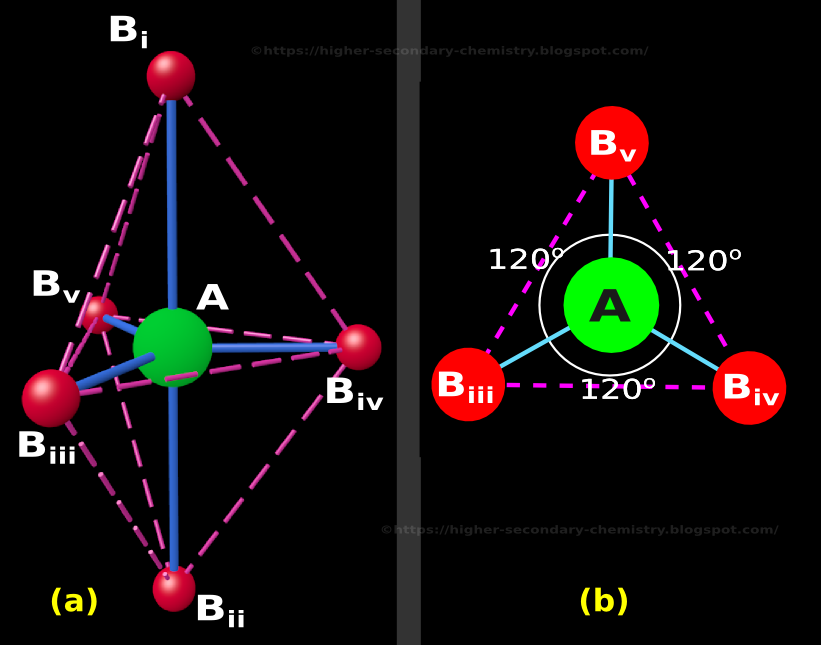
Higher Secondary Chemistry Chapter 4.15 The Trigonal Bipyramidal Structure in VSEPR Theory
AB2U 3. Molecular Geometry. AB 6, AB5U, and AB4U 2. Some examples of molecules with this geometry are: SF 6, SeF 6, SCl 6, etc. These molecules are examples of central atoms with six bonding pairs of electrons. Molecules are octahedral and nonpolar when all six substituents are the same. If the six substituents are not the same polar.
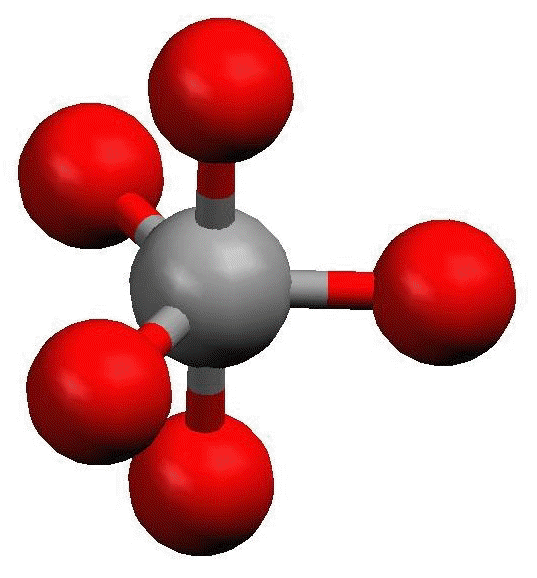
Molecular Modeling 1 Chem Lab
Introduction. Main medical applications of In III complexes are currently related to the use of the isotope 111 In (electron capture with gamma emissions of 171 and 245 keV, t 1/2 = 67.4 h) in diagnostic radiopharmacy, 1-4 where it is frequently used for the imaging of infection and inflammation sites. Additionally, 111 In is under discussion for cancer therapy, since it also emits Auger.

Trigonal Bipyramidal Molecular Geometry/Shape and Bond Angles YouTube
3D rotating molecule - Trigonal Bipyramidal. created by North Carolina School of Science and Mathematics copyright © 2013 North Carolina School of Science and.
Trigonal bipyramidal structure Science, Chemistry, Molecular Geometry ShowMe
The base angles are still 180°, 120°, and 90° while the tweaked angle will now be slightly less in each case due to the extra repulsion from the lone pair. POLARITY: POLAR - The lone pair electrons throw off the perfectly cancelling symmetry of the five trigonal bipyramidal regions thus making the overall molecule polar.

Molecular structure of 1showing the trigonal bipyramidal polyhedron at... Download Scientific
In this video we'll look at the Trigonal Bipyramidal Molecular Geometry and Bond Angles. We'll use the example of PCl5 to understand the molecular shape..
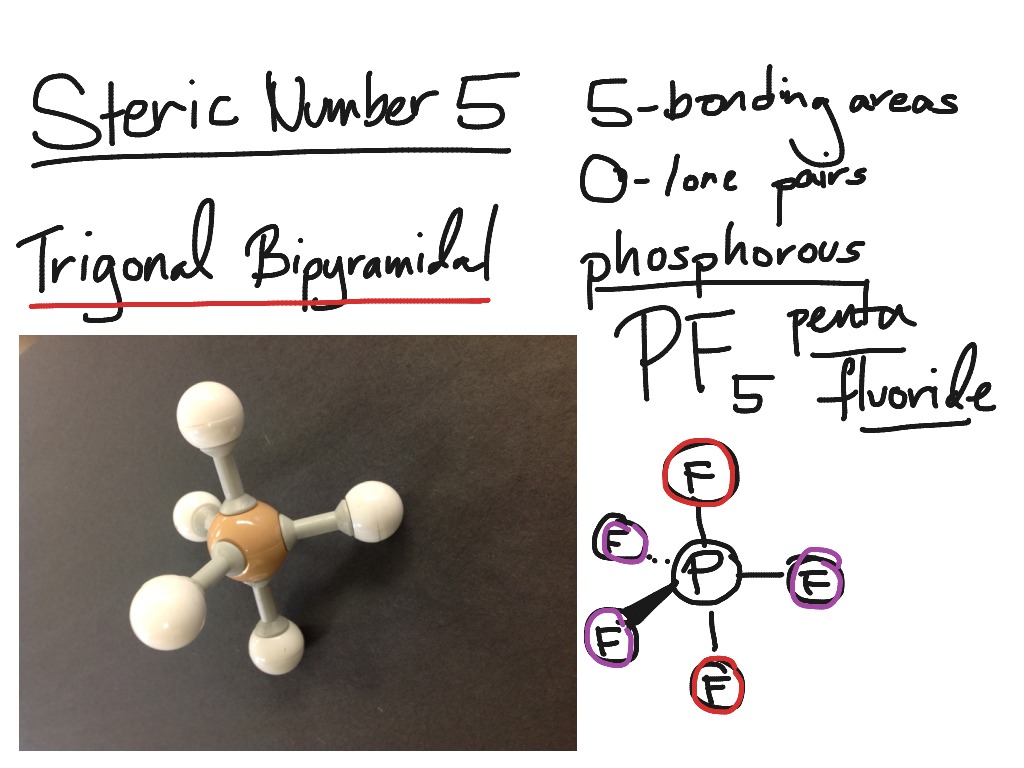
Apicophilicity Trigonal Bipyramidal Molecular Geometry Structure Molecule Monomer, PNG
The CTB geometry is identified by donor atoms taking up one apex and three equatorial positions of a trigonal bipyramid (TBP) (Fig. 1).The remaining two coordination sites R 1 and R 2 form the top of a distorted Y shape around the opposite apex, effectively adding a capping atom to a TBP with a R 1 MR 2 angle of 50-80°. The R 2 ML angle (Fig. 1) ideally is close to 180° but in practice can.
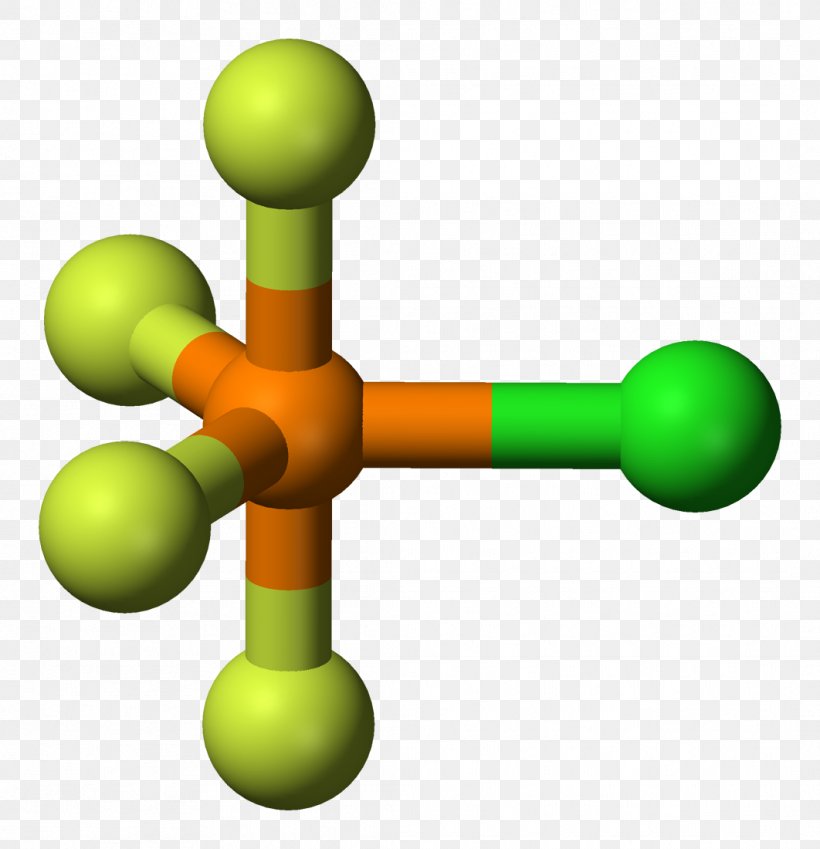
Trigonal Bipyramidal Molecular Geometry VSEPR Theory Planar Molecule Ax Transparent PNG
Trigonal bipyramidal molecular geometry. In chemistry, a trigonal bipyramid formation is a molecular geometry with one atom at the center and 5 more atoms at the corners of a triangular bipyramid. [1] This is one geometry for which the bond angles surrounding the central atom are not identical (see also pentagonal bipyramid ), because there is.

Trigonal Bipyramidal Molecular Geometry Download Free 3D model by orgoly [8a7db40] Sketchfab
Synthetic chemistry enables a bottom-up approach to quantum information science, where atoms can be deterministically positioned in a quantum bit or qubit. Two key requirements to realize quantum technologies are qubit initialization and read-out. By imbuing molecular spins with optical initialization and readout mechanisms, analogous to solid-state defects, molecules could be integrated into.

Trigonal Bipyramidal Geometria Molecular, Trigonal Plana Geometria Molecular, Pentagonal
The stereoisogram approach, which has originally been developed to rationalize organic stereochemistry (Fujita in J Org Chem 69:3158-3165, 2004; Fujita in Tetrahedron 62:691-705, 2006; 65:1581-1592, 2009), is extended and applied to inorganic stereochemistry by using trigonal bipyramidal compounds as examples. The point group D 3h of a trigonal bipyramidal skeleton is extended into the.

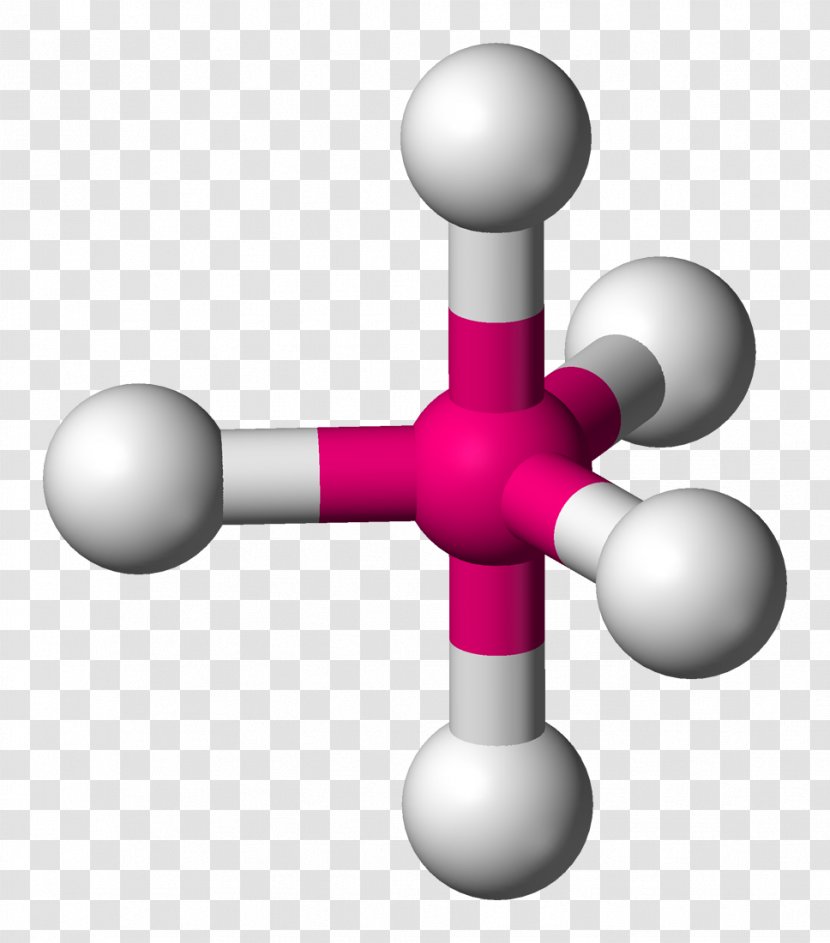
Seesaw Molecular Geometry VSEPR Theory Trigonal Bipyramidal Molecular Geometry, PNG, 1100x858px
Trigonal Bipyramidal Geometry. Trigonal bipyramidal arrangement of 5 regions of high electron density (white). Three regions of high electron density point at the corners of an equilateral triangle. One region of high electron density is directly above the plane of the triangle, and one is directly below the plane. A trigonal bipyramidal.
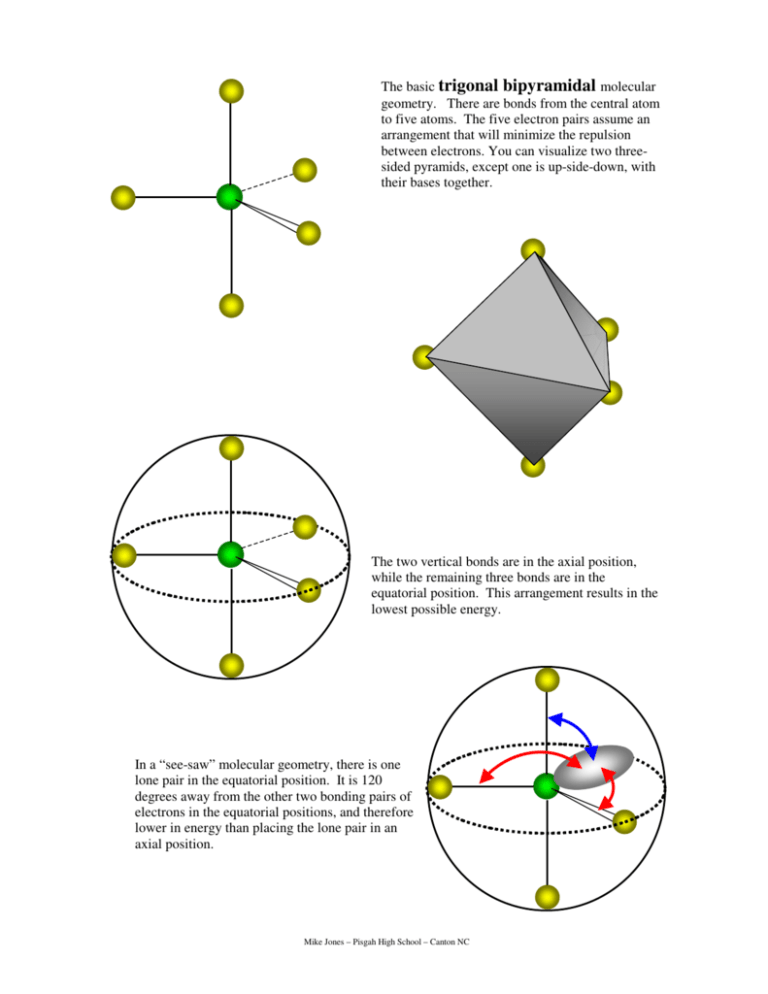
The basic trigonal bipyramidal molecular
Triangular bipyramid. In geometry, the triangular bipyramid is the hexahedron with six triangular faces, constructed by attaching two tetrahedrons face-to-face. The same shape is also called the triangular dipyramid [1] [2] or trigonal bipyramid. [3] If these tetrahedrons are regular, all faces of triangular bipyramid are equilateral.