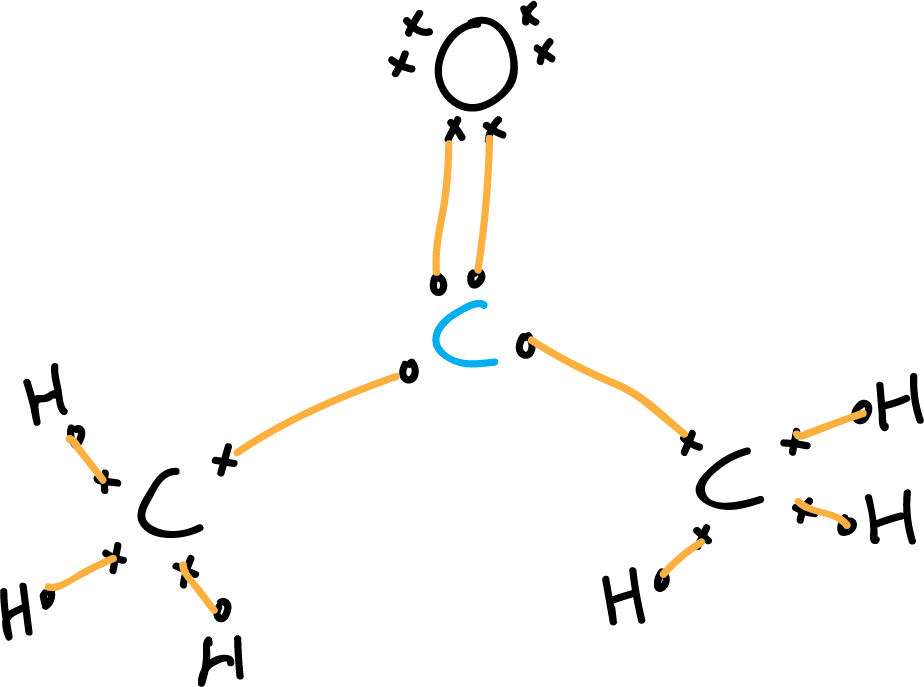
Lewis structure of acetone CH3COCH3 Chemistry Online
The two-dimensional torsional potential function for the methyl internal rotors in acetone, CH3COCH3, was derived by a global least-squares fit to spectroscopic data only. The data consisted of.
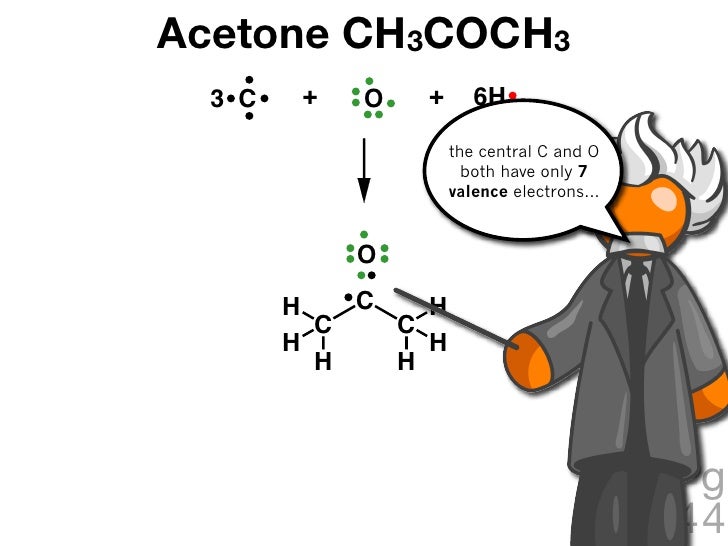
Acetone CH3COCH3 3 C
Acetone CAS 67-64-1 for analysis EMSURE® ACS,ISO,Reag. Ph Eur - Find MSDS or SDS, a COA, data sheets and more information.
.jpg)
Acetone CH3COCH3
3. Compute Mass of Each Element. Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass of each element in CH3COCH3: Molar Mass (g/mol) C (Carbon) 3 × 12.0107 = 36.0321. H (Hydrogen) 6 × 1.00794 = 6.04764. O (Oxygen)
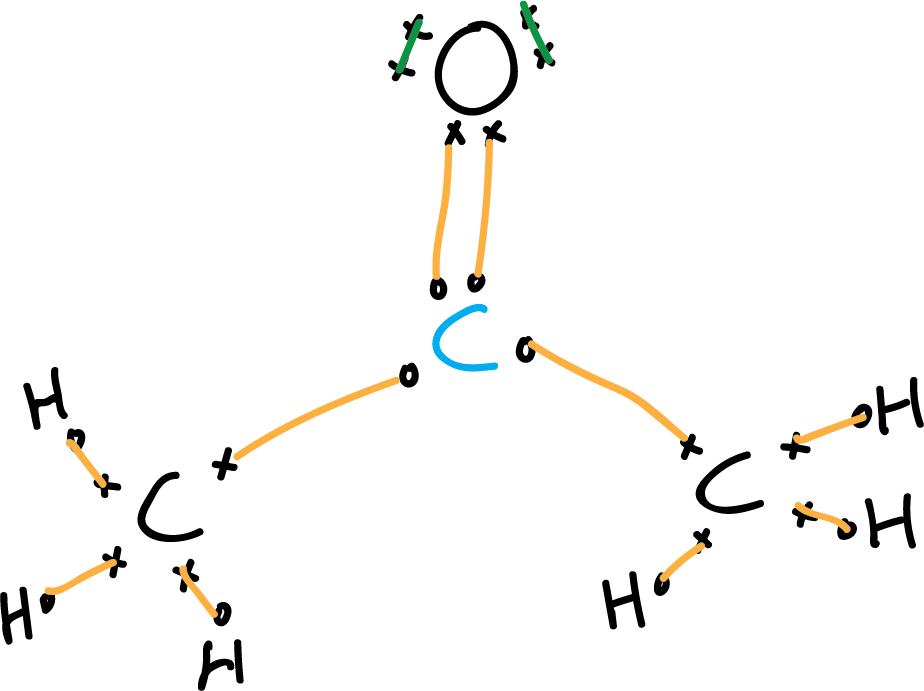
Lewis structure of acetone CH3COCH3 Chemistry Online
Acetone | CH3COCH3 or CH3-CO-CH3 or C3H6O | CID 180 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.
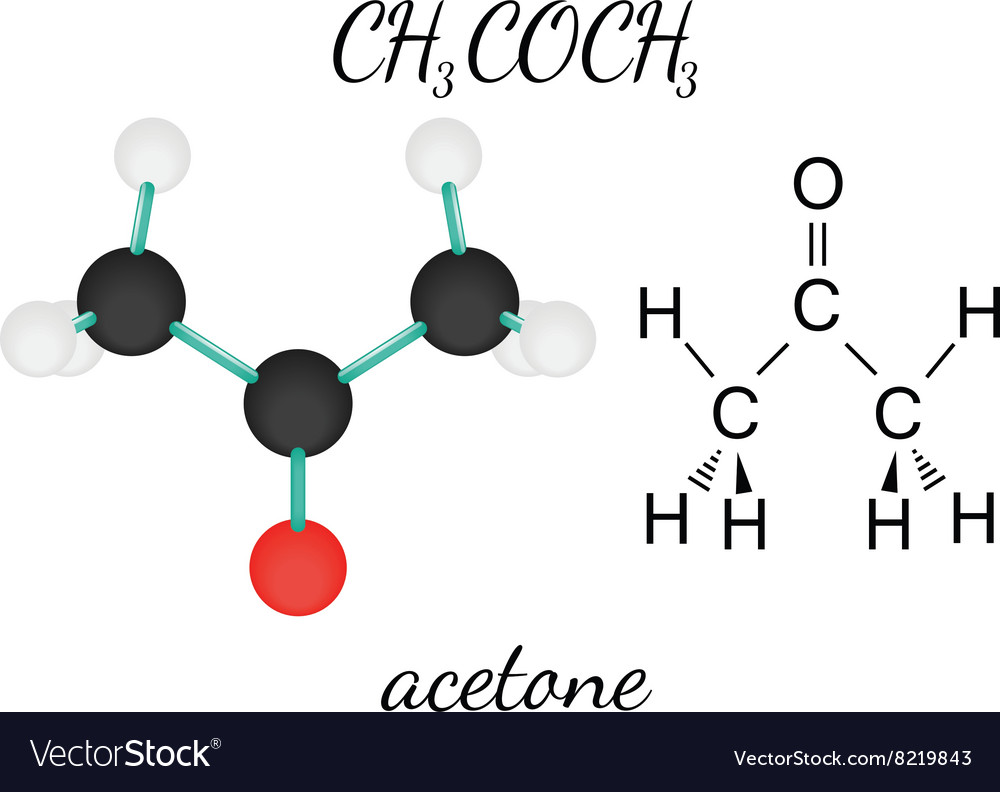
Ch3coch3 acetone molecule Royalty Free Vector Image
6m. Magnetic Properties of Complex Ions: Octahedral Complexes. 11m. Acetone, CH3COCH3, is a nonelectrolyte; hypochlorous acid, HClO, is a weak electrolyte; and ammonium chloride, NH4Cl, is a strong electrolyte. (a) What are the solutes present in aqueous solutions of each compound?
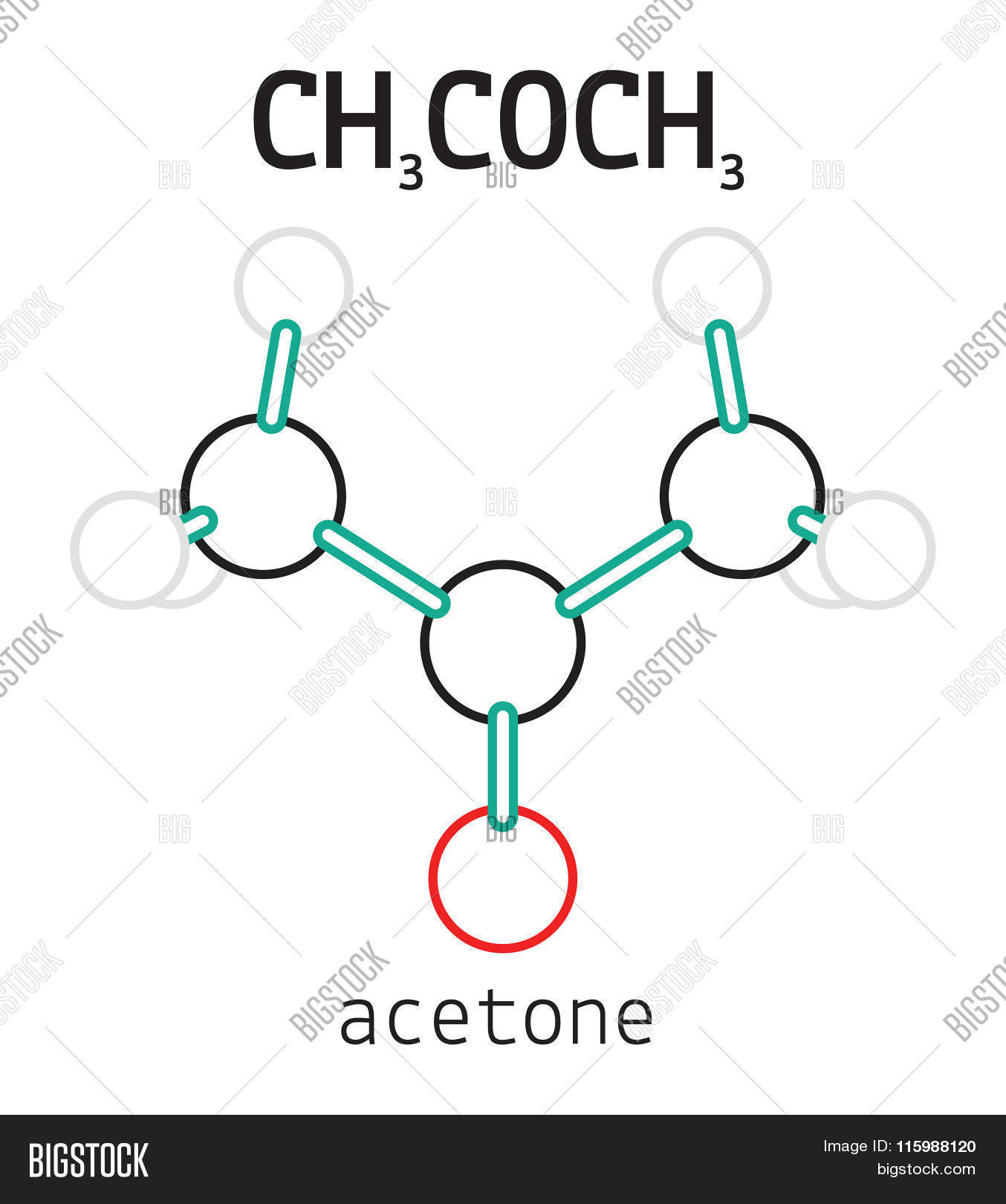
CH3COCH3 Acetone Vector & Photo (Free Trial) Bigstock
Multiphoton ionization and fragmentation studies at 355 nm have been performed for acetone molecule using time-of-flight mass spectrometry, showing a peak at =29 (COH) in the case of CH =30 (COD, which provided another evidence for partial isomerisation of the keto form to the enol form Ref. [6]. Further observation in an experiment using 355.
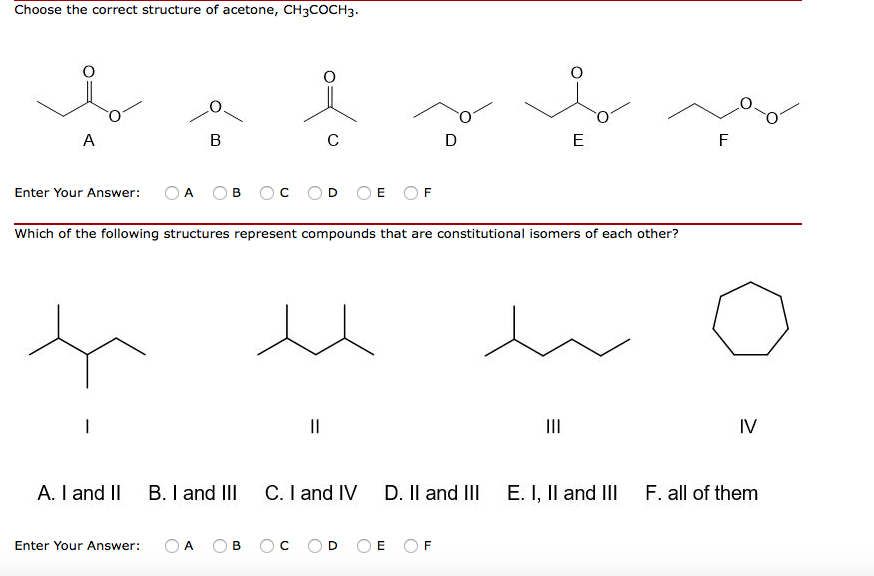
Solved Choose the correct structure of acetone, CH3COCH3
Taking into account the molecular structure and the ionization energies of acetone along with its isomers (Figure 1 and Table 1), ice mixtures of methane (CH 4)-acetaldehyde (CH 3 CHO) and d 4-methane (CD 4)-acetaldehyde (CH 3 CHO) were explored. The choice of d 4-methane (CD 4) in isotopic experiments assists in the elucidation of distinct reaction pathways based on mass shifts and the.

Aceton CH3COCH3
You can make ads in the Engineering ToolBox more useful to you! Acetone (2-propanone), CH 3 -CO-CH 3 , is a clear colorless liquid with a characteristic fruity and sweetish odor. It is flammable and vapors are heavier than air. Acetone is toxic in high doses. Acetone occurs naturally in plants, trees, forest fires, vehicle exhaust and as a.

ACETONE VM CH3COCH3 PPHC CÔNG TY TNHH HKCHEM
3. The naming is done on the basis of the number of carbons present in the parent chain.The first member of each group is "form-". However we do not have a "formone" as ketonic groups are non-terminal.The CHX3COX− C H X 3 C O X − groups are called "acetyl" groups (as they already contain 2 atoms of carbon) and thus the very first compound.
[Solved] Acetone, CH3COCH3, is a common laboratory solvent. It is usually... Course Hero
The Institute of Physics (IOP) is a leading scientific society promoting physics and bringing physicists together for the benefit of all. It has a worldwide membership of around 50 000 comprising physicists from all sectors, as well as those with an interest in physics.

Acetone [CH3COCH3] [CAS_67641] Clear Liquid 55 Gallon 353 Lb Drum Wintersun Chemical
Acetone-13 C 1 is a complex organic molecule with two internal methyl (-CH 3) rotors having relatively low effective barriers to internal rotation of about 249 cm − 1.This leads to two low-lying torsional modes and five internal rotation components resulting in a dense and complicated spectrum. In this study, measurements of acetone-13 C 1 were performed with an isotopically enriched sample.
.jpg)
Acetone CH3COCH3
Acetone is a highly flammable organic compound. The formula of Acetone is CH3COCH3. It is found in the exhaust from vehicles, plants and forest fires. Acetone is a powerful solvent that is used as nail polish remover . To Learn about the structure of Acetone, its preparation, chemical, physical properties, uses and FAQs. Visit BYJU'S for more content.
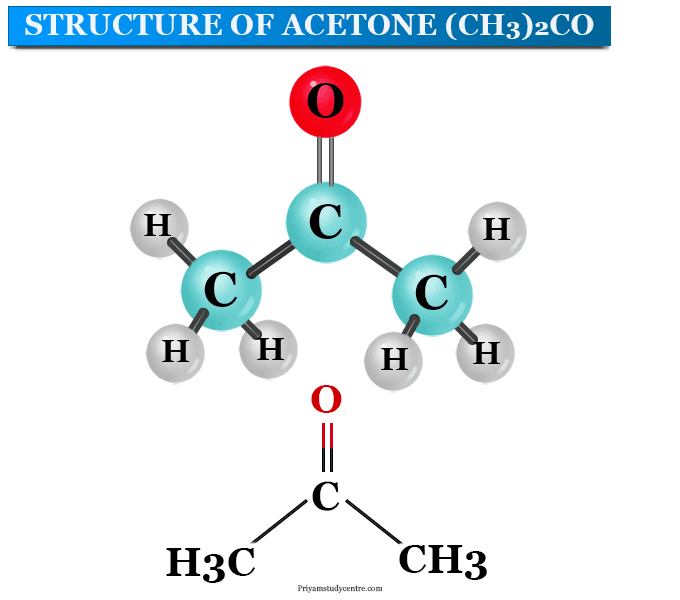
Acetone Structure
Acetone ACS reagent; CAS Number: 67-64-1; EC Number: 200-662-2; Linear Formula: CH3COCH3; find Sigma-Aldrich-V000187 MSDS, related peer-reviewed papers, technical documents, similar products & more at Sigma-Aldrich

Acetone (AR) CH3COCH3 Vật Tư Bách Khoa
Acetone (2-propanone or dimethyl ketone) is an organic compound with the formula (CH 3) 2 CO. It is the simplest and smallest ketone (>C=O).It is a colorless, highly volatile and flammable liquid with a characteristic pungent odor.. Acetone is miscible with water and serves as an important organic solvent in industry, home, and laboratory. About 6.7 million tonnes were produced worldwide in.
Mua Axeton acetone CH3COCH3 tinh khiết 500ml giá rẻ nhất TecKi.Vn
Magnetic Properties of Complex Ions: Octahedral Complexes. 11m. Acetone, CH3COCH3, is a nonelectrolyte; hypochlorous acid, HClO, is a weak electrolyte; and ammonium chloride, NH4Cl, is a strong electrolyte. (a) What are the solutes present in aqueous solutions of each compound?

Jual Acetone CH3COCH3 for analysis Merck 1.00014.2500 Cap. 2,5L di Seller Anes Medika Center
Acetone (CH3COCH3): Acetone also has a polar molecule due to the presence of the carbonyl group (C=O), which results in a partial positive charge on the carbon atom and a partial negative charge on the oxygen atom. While acetone exhibits dipole-dipole interactions due to its polarity, it lacks hydrogen bonding sites like those present in water.